Occurence and survival of viruses in composted human feaces
2 Pathogenic viruses in human faeces
2.1 Introduction
Over 130 different types of pathogenic viruses are shed in human faeces (Table 2.1). Based on their pathogenesis, such viruses can be classified as enteropathogenic viruses, for which the gastrointestinal system is the principal site of infection (e.g. astroviruses, caliciviruses and rotaviruses) and non-enteropathogenic viruses, which can occur in the intestinal tract but not in association with gastroenteritis (e.g. most adenoviruses, enteroviruses and hepatitis A/E viruses). Other viruses (e.g. enteric coronaviruses, certain "small round viruses" and "parvovirus-like" agents) have been detected in faeces of patients affected by gastroenteritis, but their pathogenicity has not been proved yet /36/.
Contamination with urine or blood may lead to the occurrence in faeces of other important human pathogenic viruses, like the human immunodeficiency virus (HIV) and the hepatitis B virus (HBV). However, such viruses are not of particular relevance in relation to composting of human faeces, due to their sporadic occurrence in human faeces, their poor survival in the environment, and their route of transmission (i.e. parenteral). These factors minimize the risks of human exposure to these viruses via the production and usage of composted human faeces.
This chapter describes the most important viral pathogens occurring in human faeces (section 2.2). Particular emphasis was given to review aspects relevant to composting of human faeces, like their occurrence in human faeces (section 2.3), resistance to physical-chemical factors (section 2.4) and infective dose for humans (section 2.5).
Table 2.1.
Main pathogenic viruses occurring in human faeces. Modified from Hurst /37/.
Virus group |
No. of serotypes |
Disease |
|
Adenovirus |
47 |
Respiratory disease, conjunctivitis, gastroenteritis |
|
Astrovirus |
8 |
Gastroenteritis |
|
Calicivirus |
2 |
Gastroenteritis |
|
Enterovirus |
Poliovirus |
3 |
Paralysis, meningitis |
Coxsackievirus A |
24 |
Herpangina, respiratory disease, meningitis, paralysis |
|
Coxsackievirus B |
6 |
Myocarditis, pericarditis, congenital heart anomalies, rash, diarrhoea, meningitis, respiratory disease, pleurodynia |
|
Echovirus |
31 |
Meningitis, respiratory disease, pericarditis, myocarditis, rash, diarrhoea, fever |
|
Enterovirus 68-71 |
4 |
Meningitis, respiratory disease |
|
Hepatitis A virus |
1 |
Infectious Hepatitis |
|
Hepatitis E virus |
1 |
Infectious Hepatitis |
|
Rotavirus |
4 common 10 total |
Gastroenteritis |
|
2.2 Major pathogenic viruses occurring in human faeces
This section reviews the basic morphological, biological, clinical and epidemiological traits of the principal viral pathogens occurring in human faeces. Data were obtained from relevant books /23,38), review articles in scientific journals /37/, websites /39,40/ and WHO documents /41,42/.
Adenoviruses (family Adenoviridae) are non-enveloped double-stranded DNA viruses with an icosahedral shape, a diameter between 80 and 110 nm, and fiber appendages protruding from the vertices of the icosahedral viral capsid. These viruses are unusually stable to physical and chemical agents, and adverse pH conditions. They tolerate a pH range of 5.0-9.5 and temperatures between 4 and 36EC. Heating above 56oC disrupts the virus capsid, causing inactivation. These resistance properties confer to adenoviruses the ability to survive for long periods outside of the host cells. Furthermore, these viruses are among the most persistent in sewage treatment systems.
Adenoviruses are divided into subgroups A-F, with members of the A-E subgroups causing respiratory infections and members of the F sub-group causing enteric infections. Infections are common and affect primarily children. Symptoms of infections and epidemiological patterns vary between sub-groups and even for different species. Although some adenoviruses can cause gastroenteritis in young children (i.e. serotypes 40 and 41) or genital infections (i.e. serotypes 19 and 37), these organisms are most frequently observed to cause respiratory and eye infections. The duration of the illness is generally 7-8 days. The predominant symptoms include fever, throat pain, headache, abdominal pain and conjunctivitis. Asymptomatic infections occur with long-term virus shedding from the respiratory or enteric tracts.
Adenoviruses are endemic worldwide. In temperate regions, they show a seasonal incidence, with highest incidences in the fall, winter and early spring. Transmission generally occurs by the respiratory route (inhalation of aerosols) and sometimes by the faecal-oral route. Transmission by recreational water (e.g. swimming-pools or other recreational waters) has been documented. An infected person can excrete the virus from the respiratory tract. However, the virus can disappear from respiratory secretions after a short time and can be found in faecal specimens, sometimes for extended periods.
Members of the family Astroviridae are small (26-32 nm of diameter), non-enveloped RNA viruses. The name of this group of viruses derives from their star-like appearance observed by transmission electron microscopy after negative staining. These viruses are resistant to pH 3 and can survive at 60°C for 5 min.
Astroviruses are primarily associated with mild gastroenteritis in infants and young children, although elderly, hospital patients and immunocompromised individuals can also be affected. These viruses display many of the epidemiological and clinical features of rotaviruses, but are not as common and not as virulent. The illness has a normal duration of 2-3 days. Viral shedding may begin a day before symptoms are seen and continue for several days after the diarrhoea has stopped.
Astrovirus infection occurs worldwide and accounts for 2-8% of cases of diarrhoea in infants, second only to rotavirus as a cause of childhood diarrhoea. Like rotaviruses, astrovirus infections occur throughout the year with peaks in the winter months. Person to person spread by the faecal-oral route is the main route of transmission. Outbreaks tend to occur where children are in close contact, as in day-care centres, kindergartens and paediatric wards.
Enteroviruses form a genus within the family Picornaviridae, which includes important animal (e.g. foot and mouth disease) and human (e.g. hepatitis A) pathogenic viruses. The genus Enterovirus was traditionally divided into three groups (polioviruses, coxsackieviruses and echoviruses). Enteroviruses are small (22-30 nm), non-enveloped, RNA viruses that are highly resistant to the conditions prevailing in the gut, like acid pH, proteolytic enzymes and bile salts. They are stable at acid pH (3-5) for 1-3 hours.
The clinical syndromes caused by enteroviruses include neurological disease, cardiac and muscular disease, rash, respiratory disease, ocular disease and neonatal disease. For all members of the group, sub-clinical infection is far more common than clinically manifest disease. Certain serotypes are more frequently associated with epidemics involving a specific syndrome. However, the same serotypes may cause different clinical manifestations or produce no symptoms.
The most famous enteroviruses are the Polioviruses, of which there are 3 distinct types. These viruses cause poliomyelitis, an acute infection of the central nervous system, which can result in flaccid paralysis. The disease can have several forms: abortive poliomyelitis, a minor illness with fever, malaise, drowsiness, nausea, vomiting, constipation and sore throat, non-paralytic poliomyelitis or aseptic meningitis with the additional symptoms of a stiff neck and back, and paralytic poliomyelitis marked by flaccid paralysis. Non-paralytic illness is short-lived and patients recover without permanent damage. Paralytic disease occurs in about 1% of infections and symptoms may persist for months, with residual paralysis lasting years. Mortality among the paralytic cases varies between 4 and 10% depending on the virulence of the virus, the degree of medical assistance and the age of the patient. Recrudescence of paralysis and muscle wasting sometimes appears decades later in some persons who had paralytic poliomyelitis.
Vaccines against polioviruses have been available since the 1950s and efforts are now in progress to completely eradicate poliomyelitis and their causative viruses from the human population by the year 2005. Such global eradication seems achievable because regional eradication has already been achieved in the Western Hemisphere and Western Europe. However, there are no vaccines against the other enteroviruses.
Children are the prime targets of enteroviruses and serve as a vehicle for their spread. It has been calculated that more than 90% of children living under poor sanitary and socio-economic conditions experience infections with a number of the locally prevalent enteroviruses before they reach the age of 5 years. When infection is delayed to older childhood and adult young life, the incidence of paralytic poliomyelitis rises, together with the frequency of the most severe manifestations associated with other enteroviruses.
Almost all enteroviruses can be recovered from the oropharynx and intestine of individuals infected either clinically or sub-clinically. They are generally shed for a month or more in stool of infected individuals. Faecal contamination is the usual source of infections. However, droplets or aerosols from coughing or sneezing also can be a source of direct or indirect contamination for some enteroviruses.
Enteroviruses are found in all parts of the world. Climate is an important factor influencing the circulation and prevalence of these viruses. In tropical and semitropical regions, they are widely distributed throughout the year. In temperate climates, they are rarely present in the winter and are encountered far more commonly during summer and autumn.
Because enteroviruses are shed in faeces and respiratory secretions, and are relatively stable in sewage and water, it is assumed that they are transmitted by faecally contaminated water. Transmission by faecally contaminated water is likely to be one of the main routes for transmission under conditions of poor sanitation and crowding. However, the epidemiological evidence for waterborne transmission is weak despite years of surveillance for these viruses in populations.
The virus causing hepatitis A (HAV) is a small (27 nm), non-enveloped RNA virus with an icosahedral shape. HAV belongs to the same family of enteroviruses (Picornaviridae) and has similar morphological and biological characteristics to these viruses. HAV was previously classified as enterovirus 72, but it is genetically distinct from enteroviruses and is now in a separate genus called Hepatovirus. HAV is extremely resistant to degradation by environmental conditions, as demonstrated by its occurrence in freshwater, seawater, wastewater, soil, marine sediment and oysters. HAV has been found to be more resistant than some other enteric viruses to biosolids and wastewater treatment processes and to persist for as long as a 6 month in sewage-contaminated groundwater. The virus is highly resistant to heat (70° C for 10 min) and acid treatment (pH 1 for 2 h).
Hepatitis A is an acute self-limited disease accounting for approximately 1.4 millions cases in the world per year. The actual burden of disease is probably much higher due to inadequate recognition and reporting. The predominant symptoms are anorexia, jaundice, nausea and vomiting. The symptoms are highly age dependent, with adults and children over 5 years being markedly more susceptible to jaundice compared with children less than 5 years. Duration and seriousness of the disease varies from 1-2 weeks of mild illness to 6-9 months of severely disabling. Mortality rates for hepatitis A are generally less than 1% and death occurs primarily in older people. HAV can be shed before the onset of symptoms and the shedding can continue up to 3 months after resolution of the symptoms.
HAV infections account for 20-25% of clinically apparent hepatitis cases worldwide. The virus is transmitted by the faecal-oral route, either directly by person-to-person or indirectly by ingestion of contaminated food (e.g. shellfish) and water. HAV can occur both sporadically and epidemically. Epidemics are uncommon in developing countries, where children are infected early in life and adults are generally immune. In developed countries Hepatitis A is still common and often occurs as common source outbreaks due to faecally contaminated food and water. The largest documented outbreak of Hepatitis A resulted in 300,000 cases of illness in Shanghai, China in 1988 and was caused by consumption of faecally contaminated clams.
Although not widely used, an inactivated vaccine against HAV has been available since 1995. Other prevention and control measures based on sanitation and hygiene continue to be the main barriers to transmission.
The agent causing hepatitis E (HEV) is a non-enveloped RNA virus of 32-34 nm. The virus is classified was previously within the family Caliciviridae, but because of genetic and replication differences, it is now unclassified and is likely to be placed in a unique virus family. Compared to HAV, HEV is less stable in harsh environmental conditions like high salt concentration or repeated freeze-thawing. The virus is more susceptible to heat than is HAV.
Hepatitis E has so far been observed almost exclusively in developing countries. Different strains of HEV occur in different parts of the world, with at least 4 main ones: (1) South-East, North and central Asian, Mexico, United States, and Taiwan. Individuals between 15 and 40 years of age are the most frequently affected. The disease closely resembles that described for hepatitis A, although bilirubin levels tend to be higher, and jaundice deeper and more prolonged. The mortality rate is 0.5-3%, but it can be extremely high for pregnant women (10-20%). HEV has been detected in stools 14 days after the onset of jaundice and persists for about 2 weeks. The infection is usually sub-clinical in children. As for hepatitis A, the disease does not progress to chronic hepatitis.
Outbreaks and sporadic cases of HEV have occurred over a large geographic area, most notably in regions with poor sanitation. Outbreaks of hepatitis E are more common in regions with hot climates and are rare in temperate climates. Most HEV outbreaks are due to faecally contaminated drinking water, but food-borne epidemics (raw or uncooked shellfish) have also been reported. Person-to-person transmission appears to be uncommon, perhaps because of the relatively low virus levels in faeces of infected persons.
Epidemic Hepatitis E was first identified in India, and it also occurs in the Middle and Far East, in northern and western Africa, the central Asian Republics of the former Soviet Union, in China and Hong Kong. Both epidemic and sporadic cases of HEV have been reported from southeast and central Asia, the Middle East, northern and western Africa and North America (Mexico). Sporadic cases of Hepatitis E occurring in non-endemic regions have been associated with travel to endemic regions. Recent evidence for the existence of HEV strains in animals (swine, rates, cattle, chickens, etc.) that resemble human HEV strains also raises the possibility of zoonotic transmission as the source of sporadic human cases in non-endemic areas. Experimentally, swine HEV infects primates and human HEV infects swine.
2.2.6 Norwalk virus and other human caliciviruses
The Norwalk virus is the prototype of a group of so-called "small round structured viruses" which are now classified as members of the family Caliciviridae on the basis of their nucleic sequence. Members of the family are non-enveloped contain single-stranded RNA surrounded by a capsid with cup-shaped surface structures. These viruses are generally associated with gastroenteritis in humans.
The human caliciviruses are genetically diverse. They are divided into three major groups, the Norwalk-Like Viruses (NLVs), which are subdivided into two major subgroups, GI and GII, and the Sapporo Viruses. There is genetic diversity within these groups and several different subgroups have been identified. Caliciviruses are endemic in human populations worldwide and there are distinct genetic subgroups that predominate regionally and over time. Norwalk Virus belongs to the GI group of human caliciviruses and these are no longer prevalent in Europe and North America. The GII NLVs are the predominant epidemic caliciviruses in these regions.
The Norwalk viruses are relatively stable. They can survive at pH 2.7 for 3 h, heat at 60°C for 30 minutes and drying on surfaces. The persistence Norwalk Viruses in water, wastewater and soil is similar to that of some other enteric viruses, such as poliovirus and MS2 (a male-specific RNA bacteriophage of E. coli or colipage). Information on the survival of human caliciviruses in faeces, sewage and other media are limited because these viruses infect only humans and no laboratory hosts, such as cell cultures or experimental animals are available.
The Norwalk group of viruses tends to infect older children and adults. The illness consists of an explosive episode of nausea, vomiting, diarrhoea and abdominal cramps, some times accompanied by headache, sore muscles and low-grade fever. Symptoms are usually last 12-48 hours for Norwalk virus and for 1-3 days for other human caliciviruses. The shedding of viruses declines after the onset of illness but can persist at declining levels for 1 to 2 weeks.
The Sapporo-like caliciviruses cause illness primarily in children and are endemic worldwide. Most children are infected with at least one calicivirus before leaving primary school. Outbreaks occur mainly in nursery schools and kindergartens, but also in day-care centres, orphanages, maternity hospitals and schools.
The Norwalk-like virus group is one of the major causes of gastroenteritis in adults worldwide. Approximately 40% of outbreaks of gastroenteritis in adults the USA have been attributed to this virus. Common-source outbreaks frequently occur via faecal contamination of water or food (e.g. shellfish and salads). Outbreaks frequently occur in camps, schools, nursing-homes and cruise ships. Because some caliciviruses of cattle and swine are genetically closely related to human caliciviruses, there is now suspicion that zooonotic transmission is possible and deserves consideration in elucidating the natural history of these viruses.
Rotaviruses are members of the family Reoviridae. The family also includes reoviruses, which are commonly found in human stool, but are not associated with gastroenteritis or other illnesses. Rotaviruses are 60-80 nm in diameter, non-enveloped, contain 11 segments of double-stranded RNA viruses surrounded by a protein core and two capsid layers. This three-layered structure of virus proteins causes particles to have the typical appearance of a wheel with spikes. Rotavirus survives at 60° C for 30 minutes. The virus is stable at acid pH (3.0 - 3.5) and can survive for months outside of the host at temperatures between 4 and 20°C.
Rotaviruses are the major cause of infantile acute diarrhoea in children (95% of children worldwide are infected by age 3 to 5). The disease is a major cause for childhood mortality in Africa, Asia and South America. The infection occurs usually in children between the ages of 6 months and 24 months, with the peak around 12 months. Vomiting generally precedes diarrhoea, which lasts for 4-5 days and can lead to severe dehydration. Asymptomatic infection is the rule in newborns and is quite common in older children and adults, although outbreaks in adult populations have been reported. The virus is shed in stool for as long as 10 days after the onset of symptoms. The levels of rotavirus shedding can be as high as 1011-1012 virus particles per gram of stool.
Rotaviruses are major causes of disease worldwide. In temperate regions, infections are most frequent during the winter and early spring months, with high incidence in day-care settings. Rotaviruses are mainly transmitted by the faecal-oral route, although some authors have reported their presence in respiratory tract secretions and other body fluids. Because of their stability in the environment, transmission can occur through ingestion of contaminated water or food or contact with contaminated surfaces.
A rotavirus vaccine was released in the late 1990s, but serious complications of intestinal blockage were reported in enough immunized children that the vaccine was withdrawn from the market. Currently, rotavirus infection and illness must be prevented by adequate sanitation and hygiene and controlled in ill persons by adequate rehydration, supportive care and prevention of spread to other susceptible persons.
2.3 Occurrence in human faeces
Our literature search provided no data on the occurrence of pathogenic viruses in human faeces collected for composting. Only few data exist on the occurrence of pathogenic viruses in pit latrines. A study conducted in poor areas of Texas in 1953 reported the occurrence of both polioviruses (16 out of 220 samples) and coxsackieviruses (10 out of 63 samples) in pit latrines /43/. More recently, an investigation on faecal material stored in pit latrines in Botwsana reported concentrations of enteroviruses from 0.3 to 1.5 PFU per gram, and rotaviruses from 90 to 727 virions per gram /44/.
The concentration of enteric viruses in sewage may be indicative of their occurrence in human faeces collected for composting. Virus concentrations of 5.000 to 28.000 PFU per litre are commonly found in raw sewage /45/. Recent studies have demonstrated that adenoviruses, enteroviruses, HAV and Norwalk virus are common in raw sewage, as they can be detected in samples of 100 ml at frequencies varying between 10 to 100% of samples tested /46,47).
Epidemiological data on the incidence of enteric viruses in the population may be indicative of the occurrence of enteric viruses in human faeces. According to the incidence of human infections (see section 2.2), the distribution of enteric viruses in human faeces should undergo seasonal variations. Adenovirus, astrovirus and rotavirus are more frequent during autumn, winter and early spring, whereas enteroviruses are more common in the summer.
Geographical differences should be also taken into consideration. The HEV virus is likely to occur less frequently in faeces and sewage collected in Europe. However, recent studies have reported HEV detection in sewage in Barcelona, Spain and Washington, DC, which are non-endemic areas. Therefore, the presence of this virus in human wastes is not limited to developing countries. However, the occurrence of HEV and other enteric viruses, such as enteroviruses, HAV and rotaviruses is likely to be higher in developing countries than in industrialized countries due to less coverage of water, sanitation and protection against childhood diarrhoeal diseases in developing countries.
For equal or similar incidences in the human population, viruses faecally excreted at higher numbers and for longer periods are likely to be predominant in faeces collected for composting. HAV is excreted at densities of up to 1010 viral particles per gram and the shedding occur for at least 30 days after the onset of the disease and as long as three months /48-51/. Also rotaviruses are shed at even higher numbers (1010 to 1012 viral particles per gram), sometimes for periods up to one month /52-55/. The Norwalk-like viruses have been estimated to occur at lower densities in faeces (typically 104-106 viral particles per gram) /56/ and the shedding lasts up to two weeks after infection /57,58/. Enteroviruses are generally excreted at densities of 106 infectious units (corresponding to about 108-1010 virus particles) per gram of faeces) and their excretion time is on average 7 weeks /23/.
2.4 Occurrence in Denmark
In Denmark, only the incidence of HAV is notifiable. In the year 2000, 81 patients were notified with hepatits A, corresponding to an incidence of 1.5 cases per 100.000 inhabitants /59/. According to preliminary data of the WHO Polio Eradication Certification Process, the occurrence of enteroviruses (Coxachie-, Echo, Polio- and Enterovirus 68-71) in faecal specimens from symptomatic patients was 15% in 1998, 13% in 1999 and 23% in 2000 (personal communication from Peter Henrik Andersen, Department of Epidemiology, Statens Serum Institut, Denmark).
The numbers of diagnosed rotavirus infections reported by the major laboratories in the country were 171 out of 2,770 patients tested in 2000 (6%), and 284 out of 10,222 patients tested in 2001 (3%) (personal communication from Francois-Xavier Hanon, Department of Epidemiology Research, Statens Serum Institute, Denmark). Caliciviruses (including the Norwalk virus) account for the vast majority of outbreaks of food-borne viruses and are estimated to be responsible for over 40% of total food-borne outbreaks in the country /60/. The contribution of other enteric viruses to food-borne outbreaks can be estimated to be approximately 2-3% (personal communication from Francois-Xavier Hanon, Department of Epidemiology Research, Statens Serum Institute, Denmark).
In another Scandinavian country (Finland), rotavirus appears to be the most common diarrhoea-causing virus in young children. Rotavirus was estimated to be responsible for 54% cases among children less than five years old that were hospitalised for acute diarrhoea in the period between 1985 and 1995 /61/. Caliciviruses and astroviruses were detected in 21% and 9% of episodes of acute gastroenteritis in children less than two years of age, respectively /62,63/. Also in Sweden, rotavirus is the predominant virus among children affected by gastroenteritis, being recovered from 53% of children attending hospitals with symptoms of gastroenteritis /64/.
In Denmark, the concentration of viruses in sewage has been estimated to be 103 to 105 PFU/100 ml for enteroviruses (including HAV) and 2 to 102 PFU/100 ml for rotaviruses /65/. Similar or more likely higher concentrations can be expected to occur in human faeces destined to composting, since the concentration of enteric viruses in sewage is reduced as a consequence of dilution.
2.5 Response to physical-chemical factors
The knowledge of the response of different viruses to physical-chemical factors is essential to evaluate the survival of viruses during storage of faeces and the efficiency of composting in viral inactivation. In particular, the survival of viruses during storage and composting of faeces is closely related to their resistance properties to changes of temperature, pH and moisture occurring during the different phases of the composting process.
The principal factor affecting viral survival is temperature. In general, viruses better tolerate low temperatures than high temperatures. Surface proteins are denatured within a short time at temperatures of 55-60° C, with the result that viral particles are no longer able to bind to the host cell /66/. Enteroviruses seem to be more resistant than bacteria and parasites when exposed to heat for less than one day, whereas they are more susceptible than other microorganisms for prolonged exposure times (Fig. 2.1).
It is important to recognize that there are marked differences among different viruses in the temperatures and exposure times necessary for viral inactivation. Non-enveloped viruses, like all most important enteric viruses, are generally more heat-resistant than enveloped viruses /66/. Among enteric viruses, the most heat-resistant appears to be the HAV, for which temperatures of 60° C for 30 min or 70° C for 10 min are not sufficient for complete inactivation /41,67/. However, even HAV is inactivated by heat-treatment at 60° C for 10 hours /68/.
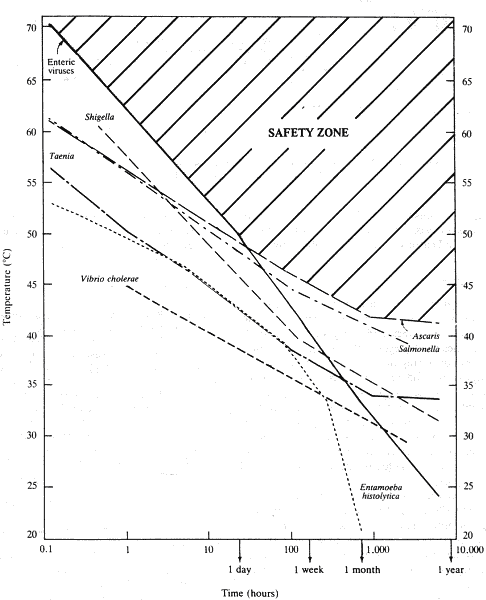
Fig. 2.1.
Effect of temperature and exposure time on enteroviruses, bacteria (Salmonella and
Vibrio choleae) and parasites (Taenia and Ascaris). According to Feachem et al. /23/.
The effects of temperature on virus survival have also been extensively studied with respect to the inactivation of viruses in animal slurries /69-77/. Table 2.4 reports the data relative to animal viruses belonging to the same families of certain human pathogenic viruses. Also in this case, differences are evident between different types of viruses, with the porcine parvovirus being particularly heat-resistant compared with the other viruses tested.
Table 2.2.
Inactivation times for animal viruses in slurry at various temperatures.
According to Bøtner /71/
Virus |
Family |
5° C |
20° C |
35° C |
40° C |
45° C |
50° C |
55° C |
Transmissible gastroenteritis virus |
Coronaviridae |
>8w |
2w |
24h |
>5h |
2h 30m |
1h |
30m |
Foot and mouth disease virus |
Picornaviridae |
>14w |
2w |
24h |
10h |
5 h |
1 h |
1h |
Porcine parvovirus |
Parvoviridae |
>40w |
> 40w |
21w |
9w |
> 19d |
5 d |
8d |
* w, weeks; d, days; h, hours; m, minutes.
Viruses are generally best preserved at physiologic pH, although some viruses tolerate a wide pH range. While most enveloped viruses are rapidly inactivated at pH 5-6, non-enveloped enteric viruses are able survive gastric acidity (pH=3)/65/, or even lower pH levels (e.g. HAV is stable at pH 1 for 2 h). Hence, low pH levels (3 to 5) are not deleterious for enteric virus survival. Most enteric viruses also are relatively stable at moderately high than high pH levels, or at least up to pH 9.5. At pH 10 and higher, the rates of enteric virus inactivation vary among the different viruses. Some viruses are not stable to pH 10 and become inactivated by >99.99% within 1 day. Other enteric viruses are stable at pH 10 for periods of weeks to months. However, most enteric viruses are inactivated by 99.99% at pH 11 or higher within 1 day or less. The low stability of enteric viruses at high pH (pH >11) is used for viral inactivation in sewage sludge by lime treatment /37/.
Enteric viruses are susceptible to dry conditions, but only if very low moisture levels are achieved (<5%). Dewatering of raw sludge was shown to effectively inactivate human polioviruses /78/. Inactivation was due to disruption of the viral capsid with consequent release of nucleic acids. However, other studies have shown that viruses can persist in relatively dry soils for long time periods. Therefore, other factors in the suspending medium or matrix besides loss of water also may contribute to virus inactivation. Insufficient data are available for comparison of the survival of different types of viruses under dry conditions in different media, as only some virus groups have been studied in certain media. However, some enteric viruses, such as HAV, have been shown to persist for long periods of time under drying or dried conditions.
2.6 Human infectivity and dose-response
The risks associated with survival of pathogenic viruses in human composted faeces are dependent on their human infectivity. Infectivity of enteric viruses and other enteric pathogens can be described quantitatively as a dose-response relationship, which is the relationship between number of virus particles ingested and the probability of resulting infection and disease in humans. As shown in Figure 2.2, the risk or probability of infection increases as the ingested dose of rotavirus to human volunteers increases.
The models that best describe such dose-response relationships are the exponential model and the Beta-Poisson model. The exponential model assumes a random distribution of organisms in the doses to which humans are exposed and that each organism has an independent and identical probability of surviving to initiate infection. The Beta-Poisson assumes that the probability of the microbe surviving to initiate infection is not a constant value but instead varies and is described by a probability distribution, the Beta-Poisson distribution.
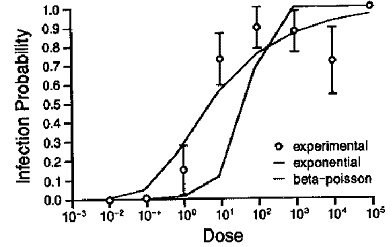
Fig. 2.2.
Dose-response relationship for infection by a human rotavirus in human volunteers with
fitted curves for exponential and Beta-Poisson models.
Most of the available data on the dose-response relationships of enteric virus infectivity for humans have been obtained from human volunteer studies. Only some enteric viruses have been studied because of the difficulties of conducting such human infection studies. However, the available data clearly indicate that most enteric viruses have a high probability of causing infection at relatively low virus doses.
The doses at which viruses have a high probability of causing infection are markedly lower than those for bacteria. For some enteric viruses, such as rotavirus and Norwalk virus, only a few viral particles need to be ingested to cause a high probability of producing disease in humans (Table 2.3). The 50% infective doses of viruses (dose at which the probability of infection is 50%) may vary between 1 and 12,000 viral particles, depending on the virus type and the state of the human exposed to the virus.
Based on dose-response relationships for infection and illness, survival of low numbers of pathogenic viruses (and parasites) in human composted faeces appears to pose a greater hazard in comparison with bacterial pathogens. However, it should be considered that, in contrast to viruses, bacteria can multiply outside of the host and their numbers can therefore increase during storage of faeces and after application of composted faeces to soil.
Table 2.3
Doses of enteric microbes infecting 50% of exposed humans (ID50 based on
excretion). Adapted from Teunis et al /79,80/.
Infectious Agent |
Approximated ID50 |
Comments |
Rotavirus |
6.1 |
Ingested in buffered water |
Echovirus 12 |
1000 |
|
Poliovirus 1 sm |
1.4 |
Attenuated vaccine strain |
Poliovirus 1 LSc2ab |
70,000 |
" |
Poliovirus 1 |
76 |
" |
Poliovirus 3 Fox |
5.5 |
Attenuated; infants |
Poliovirus 3 Fox |
5.0 |
Atenuated; premature infants |
Campylobacter jejuni |
900 |
|
Salmonella anatum |
55,400 |
|
Salmonella typhi |
858,000 |
|
Shigella flexneri |
35,900 |
|
Shigella dysenteriae |
748 |
|
Giardia lamblia |
35 |
|
Cryptosporidium parvum |
173 |
|