Occurence and survival of viruses in composted human feaces
3 Virus survival in composted human faeces
3.1 Introduction
In Denmark, there is an increased interest in using human faeces and urine for agricultural and other purposes. This interest is seen especially when new urban areas and houses are established. Traditional sewerage-based sanitation systems can cause environmental pollution and often do not utilize the nutrients contained in human excreta. An alternative to these systems is the direct recycling of human excreta using treatment systems designed to regard and employ human excreta as a resource to be used rather than as a waste to be disposed. By such systems, the nutrients contained in urine and faeces are returned to soils and plants, thereby contributing to a circular flow of nutrients (Fig. 3.1).
Fig. 3.1.
The circular flow of nutrients associated with recycling of human excreta.
An essential prerequisite for safe reuse of human faeces is the removal or destruction of pathogens by composting or other sanitation methods. This chapter describes how and to what extent viruses are inactivated during storage, production and utilization of composted faeces. The mechanisms and the efficiency of viral inactivation by composting are reviewed and evaluated on the basis of the existing literature.
3.2 Virus survival during storage of faeces
Virus survival in composted faeces depends not only on the conditions used for composting, but also on the storage conditions. Storage should therefore be viewed as part of the composting process. Faeces are collected by toilets separating faeces from urine (i.e. urine-diverting toilets) and stored into a vault beneath the toilet seat. During storage there will normally be no or very little composting effect because of anaerobic conditions and limited temperature increase. Other human waste management systems collect faeces and urine from homes either manually or by automated systems (such as vacuum tankers) and then treat it in bulk by composting or other processes /81/.
Viruses decrease in numbers during storage of faeces, due to their incapability to replicate outside of the host and susceptibility to a number of adverse environmental conditions. However, the die-off rate of viruses present in human faeces may show large variations depending on both the type of virus (see section 2.4) and the storage conditions. High pH, low moisture, microbial activity, free ammonia and high temperature are among the most unfavourable conditions for virus survival during storage of faeces.
The temperature of faeces stored in latrines generally does not significantly differ from ambient temperature /82/. Thus, the role played by temperature in the inactivation of viruses during storage of faeces appears mainly dependent on climate. Studies on sewage applied to soil indicate that viruses can persist for 23 weeks during the winter season in Denmark /83/ but for only 2-4 weeks during the summer or fall in Florida /84/. Similarly, the survival of animal pathogenic viruses in faeces has been demonstrated to be longer under winter conditions rather than summer conditions /85/.
Recent studies have demonstrated that some animal enteric viruses, including enteroviruses, can persist in faeces for a longer time, especially at low ambient temperatures. The inactivation of a bovine enterovirus in liquid cattle manure stored at 20oC was found to be only 2 log10 after 26 weeks /86/. The porcine rotavirus was demonstrated to maintain its infectivity after 32 months of storage at 10° C in original stool specimens /87/.
Among human pathogenic viruses occurring in faeces, HAV is a relatively heat-resistant virus and is inactivated more slowly than enteroviruses in a variety of media, including faeces. At lower temperatures, HAV, like other enteric viruses, is very persistent in faeces and other media. For example, HAV is inactivated very slowly in human faeces and manure kept at 5° C, with an observed reduction of only 1-2 log units after 70 days of storage in manure /88/ and about 625-1250 days in faeces /89/. At 25oC, HAV is relatively persistent in stored faeces (about 139 days for 4 log10 inactivation) when compared to poliovirus and male-specific (F+) coliphages (about 83-84 day). At 40oC, times for 4 log10 inactivation in faeces are about 20-21 days for poliovirus and F+ coliphages, and 29 days for HAV /89/.
A number of studies have investigated the survival of viruses in latrines, including urine-diverting vault latrines intended for recovery and potential reuse of both urine and faecal solids. High temperature and high pH levels are the most important physico-chemical factors affecting virus survival (see section 2.5). However, in many latrines, neither high temperature nor high pH levels are achieved. Therefore, extensive enteric microbe reductions are not achieved unless storage times are very long.
The bacteriophage Salmonella typhimurium 28B was used as an indicator to study the behaviour of human enteric viruses during storage of faeces collected by urine-diverting toilets /82/. The die-off of the phage was slow, with a reduction from 108 to 106 during 6 weeks of storage at temperatures between 20 and 35° C (Fig. 3.2). The long survival of the phage in faeces was attributed to the neutral pH conditions (7.8-8.5). Under these pH conditions, the loss of moisture observed during the study (dry weight increased from 85.2 to 96.5%) was not great enough to affect virus survival. On the contrary, it could have reduced viral inactivation by slowing down the biological process of degradation /82/.
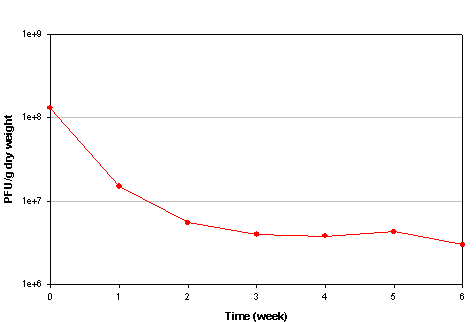
Fig. 3.2.
Mean die-off rate of the bacteriophage S. typhimurium 28B during storage of human
faeces in urine-diverting toilets. Adapted from Franzen and Scott /82/.
A similar study conducted in Vietnam suggested that the combination of high pH and low moisture have a high impact on virus survival during storage of faeces in urine-diverting latrines /90/. After 3 weeks of storage, the bacteriophage S. typhimurium 28B was reduced 8 log units in faeces with high pH (10.0 to 10.3) and low moisture content (24 to 29%). In contrast, the bacteriophage persisted for 7 weeks in faeces with lower pH (8.5 to 9.4) and higher moisture content (27 to 55%), with a reduction of 2-3 log units only. The high pH of faeces reported in this study was due to the addition of ash originating from wood and leaves /90/.
According to a summary report of various studies, Stenstrom /91/ stated that enteric microbe reduction in the material of dry latrines was mainly governed by time and high pH. Complete inactivation of indicator viruses was achieved in 6 months or less in association with the use of ash, lime or similar additives for pH elevation. Addition of only moisture-absorbing materials was shown not to ensure efficient microbe reduction.
Austin /92/ studied enteric microbe reductions in wood ash-supplemented faecal matter of urine diversion toilets in Eastern Cape province, South Africa. After 10 months of storage in a separate plastic container in the latrine, faeces contained log10 concentrations of 0-3 coliphages per gram. In additional studies, it was shown that microbial reductions were more rapid and extensive in faeces stored on the concrete floor of the latrine vault and turned weekly for aeration than in faeces stored in a closed plastic container. Coliphages were not detected after 2 months of storage at mild to cold conditions (moisture 4-8 % and pH 8.4-8.6) and when latrine faeces were subjected to a temperature of 50oC for 48 hours, while keeping the moisture content approximately the same /92/.
Moe and colleagues /93/ studied the performance and stored fecal waste quality of urine-diverting double vault latrines and solar toilets in seven communities in El Salvador. Latrine wastes ranged widely in microbial quality, probably due to high variability in the storage conditions (pH ranged from 5.1 to 12.8 and the percentage of solids ranged from 2 to 98%). Somatic coliphage concentrations varied widely and were as high as 8 log10 per gram. Temperatures of stored latrine material were 20-37.5oC (mean of 27oC), indicating that these toilets are not "true" composting systems because they do not achieve the high internal temperatures (>50oC) typical of aerobic composting. No single physical factor (pH, temperature, moisture content, or storage time) could predict microbial indicator concentration, suggesting that microbial quality is the net result of the effects of a multiple factors /93/.
Chien et al. /94/ studied the survival S. typhimurium phage in various designs of urine-diverting, double-vault latrines in Viet Nam. Phage survival was 23-154 days, with temperatures of 30-40oC, moisture contents of 25.4-58.8% and pH of 8.4-10.3. In general, microbial survival was most influenced by pH. Both pH and moisture content influenced bacteriophage when temperature was below 40oC. To achieve acceptably low levels of microbial risk, 6 months of retention for faecal materials were needed in test toilets /94/.
The available data on viral persistence in stored faecal material suggest that in temperate countries the impact of temperature on virus survival is limited to the summer months. At temperatures below 20° C, high pH values (>9.0) in combination with low moisture contents are the most important virucidal factors. Accordingly, wood ash and other substances increasing the pH levels (e.g. lime) can be used to reduce the content of viral pathogens in faeces collected for composting. Soil may be added to faeces for reducing their moisture content, although this practice does not seem to be particularly effective in viral inactivation /82/.
Aeration is another factor enhancing viral inactivation during storage of faeces. According to a study on faeces from urine-diverting toilets /91/, the die-off of viruses is higher if faeces are collected and stored in heaps and turned weekly, rather than in closed compartments. Similar evidence was provided by a study on animal waste /85/, in which a large variety of viruses (i.e. coliphage f2, picorna-, rota-, parvo-, adeno- and herpesvirus) were shown to persist for longer periods under non-aerated conditions. Consequently, the effect of aeration conditions on viral inactivation may have important implications in the design and construction of urine-diverting toilets.
3.3 Factors affecting viral survival during composting
The inactivation of viruses during composting is determined by a combination of chemical, physical and biological factors. The most important cause of viral inactivation is the heat generated during the thermophilic phase of composting. To a lesser extent, viruses are also inactivated by microbial degradation and ammonia. It should be noted that these mechanisms operate at the same time during composting. Thus, viral inactivation should be attributed to a synergistic interaction between them, rather than to each mechanism taken individually.
Environmental factors such as moisture content and pH play a secondary role in viral inactivation during composting. The optimal conditions for composting are given by pH levels of 5.5 to 8.0 and moisture contents of 40 to 60% /8,9/. These conditions are not harmful for enteric viruses by themselves (see section 2.5). Accordingly, pH and moisture can affect virus survival only by influencing the metabolic activity of bacteria and fungi and the production of ammonia during composting.
The temperature increase seen during the first phase of an optimal compost process exceeds the temperature levels needed for viral inactivation. Feachem et al. /23/ studied the effects of heat and exposure time on enterovirus survival with respect to composting of sewage sludge. According to these authors, exposure at 30° C for 3 months, at 40° C for 2 weeks, at 50° C for 1 day or at 60° C for 2 hours are sufficient to assure complete inactivation of enteroviruses, adenoviruses and reoviruses. The zone of safety described in Fig. 3.3 indicates the possible combinations of temperature and time necessary for complete elimination of enteroviruses in composted sludge.
Fig. 3.3.
The influence of temperature and exposure time on survival of enteroviruses. According
to Feachem et al. /23/.
Due to their remarkable resistance to heat (see section 2.5.1), some enteric viruses (e.g. HAV and parvoviruses) are likely to represent an exception to the safety zone described by Feachem et al. /23/. A recent study demonstrated that the bacteriophage S. typhimurium 28B is only reduced 1-2 log units after composting at 55° C for 24 hours /95/. The inactivation of certain animal enteric viruses (i.e. parvovirus) by composting requires up to 8 days at 55° C /70/. However, heat-resistant viruses are appreciably inactivated when compost facilities are operated at times and temperatures specified in accordance with current legislation (Table 3.1).
Problems may occur in the control of temperature during composting. Strauch et al. /96/ reported that it is nearly impossible in daily practical operations to constantly maintain the necessary parameters and control them. The authors proposed that composting of sewage sludge should be operated as a two-stage system, with a minimum hydraulic retention time in the system of 5 days and minimal retention time of one day in each reactor. The recommended temperatures and exposure times were either 48 hours at more than 50° C or 24 hours at 58° C. These temperature and time requirements are largely fulfilled by the current legislation on composting in different countries (Table 3.1).
Among the different systems of composting, enclosed systems ensure the best control of temperature, with horizontal reactors being preferable to vertical reactors /97/. Static aerated piles guarantee a better temperature control and therefore also a more efficient pathogen removal compared with windrow systems. The control of temperature is particularly critical in the windrow system because the temperature in the outer part of the windrow is influenced by ambient temperature. This is also true for static compost piles. Thus, the techniques used for turning must ensure that also the outer material is transported into the interior of the windrow and exposed to the temperature and time necessary for inactivation of viruses and other pathogenic organisms.
Table 3.1.
Minimum temperature and time requirements for composting in various countries.
According to Stentiford /9/ and Strauch /28/.
Country |
Minimum time/temperature requirements |
Austria |
65° C for 6 days |
Belgium |
60° C for 4 days |
Denmark (in-vessel techniques)1 |
70° C for 1 hour |
Denmark (open systems)2 |
55° C for 2 weeks |
France |
60° C for 6 days |
Germany (open windrow) |
55° C for 2 weeks or 65° C for at least 1 week |
Germany (encased windrow or in-vessel techniques) |
60° C for 1 week |
Italy |
65° C for 2-3 days |
Netherlands |
55° C for at least 2 days |
Switzerland |
55° C for at least 3 weeks or > 60° C for at least 1 week |
USA (static pile or in-vessel techniques)3 |
55° C for at least 3 days |
USA (windrows) 3 |
55° C for at least 15 days with at least 5 turnings |
1
Composting of sewage sludge for agricultural use.2 Composting of organic waste other than sludge.
3 Relative to processes to further reduce pathogens (PFRP)(see section 1.7.3).
3.3.1 Microbial degradation and enzymatic activity
Microbial breakdown is likely to play an important role in the destruction of viruses during both storage and composting of faeces. The viral capsid is composed of proteins and therefore susceptible to the action of proteolytic enzymes released by microorganisms during the biodegradation process. Unfortunately, there is a lack of data on susceptibility of viral proteins to the conditions prevailing in compost heaps. However, various studies indicate the importance of bacteria and protozoa in virus removal.
Many bacteria produce proteolytic enzymes inactivating enteric viruses, including certain bacterial species that are prevalent in the mesophilic flora during maturation of compost, for example B. subtilis /98/. Laboratory experiments have demonstrated that cell-free filtrates of bacterial cultures have an antiviral activity and that such an activity is inhibited by protease inhibitors, indicating that enzymes present in the filtrates are responsible for viral inactivation /98-100/. It also appears that viruses may serve as a nutrient source for bacteria, as indicated by the recovery in bacterial cells of labelled viral capsid proteins /98/.
In a field experiment, Poliovirus 1 was inactivated more rapidly in mixed waste than in autoclaved mixed waste and bacterium-free filtrate of raw mixed waste /99/. These results suggested a role of microbial activity in viral inactivation. The slower rates of virus inactivation in autoclaved waste could be due to disruption of antiviral bacteria and their products as well as destruction of other heat-labile constituents. The intermediate inactivation of viruses in bacteria-free filtrates of raw waste (compared to raw waste or autoclaved waste) could be due to the absence of antiviral bacteria but the continued presence of heat-labile antiviral chemicals, as well as the destruction by autoclaving of heat-labile chemicals that are protective of viruses.
In a subsequent study on the persistence of HAV in mixtures of septic tank effluent (STE) plus dairy cattle manure slurry, and in mixtures of STE plus swine manure slurry /100/, HAV was consistently inactivated more rapidly in the two types of mixed wastes than in STE alone or in a PBS control. Bacterial strains showing antiviral activity on HAV were isolated from manure. In most cases, viral inactivation was associated with bacterial production of proteolytic enzymes. However, the inactivation of HAV by some bacterial strains was not affected by protease inhibitors, suggesting that mechanisms other than enzymatic degradation could be involved in viral inactivation by bacteria. This was in accordance with a previous work documenting the antiviral activity of non proteolytic-substances produced by bacteria /98/.
Bacteria are not the only microorganisms able to affect virus survival. Predation by protozoa is a mechanism of viral removal in activated sludge /101/. However, protozoa cannot affect the survival of viruses during composting, as these organisms are killed at the high temperatures typically reached during the composting process. In urine-diverting vaulted latrines employing composting or desiccation processes, however, temperatures may not reach those attained during typical bulk composting treatment of other wastes as in static piles, windrows or mixed vessels. Under these lower temperature conditions, other microbes such as parasites and fungi could contribute to virus inactivation and biodegradation.
Destruction or loss of infectivity of viruses during composting is also due to the virucidal activity of ammonia originating from protein degradation. Various authors have demonstrated that molecular ammonia has a virucidal effect on enteric viruses /102-104/. This virucidal effect of ammonia is pH-dependent, with the anti-viral activity demonstrated primarily at higher pH levels of 8.5 and above. This is because the activity is mediated by free ammonia and not ammonium ion. The high temperatures reached during the composting process appear to be conducive for viral inactivation by this mechanism, since they enhance both production and virucidal activity of ammonia /105/.
Virus inactivation is influenced by moisture content, especially if the moisture content gets very low. Typically, composting is done at moisture content levels at which viruses are stable. It is only when compost or faecal wastes are dried or cured to low moisture levels that virus inactivation is induced. Some virus inactivation is achieved when moisture content declines to about 15%, but generally, rapid and extensive virus inactivation is not likely to be achieved until the moisture content of the material is at or below 10% /106-108/.
Virus inactivation is influenced by pH. Most viruses are stable in the pH range of 5 to 9. The extent to which viruses are inactivated above pH 9 and below pH 5 varies with the virus. Most non-enveloped enteric viruses are stable at pH levels as low as 3 and as high as 10 to 10.5. Composting is done at pH levels where viruses are stable, but some latrine wastes are supplemented with lime, wood ash or other alkaline material that may raise the pH to 10 to higher. Under these conditions viruses are likely to be inactivated due to the structural changes associated with exposure to high pH levels /109/. However, at moderately alkaline pH levels of 8.5 to 9.5, viruses are also inactivated by the presence of free ammonia (see section 3.3.3). Therefore, there can be synergistic effects of moderately alkaline pH and free ammonia in compost and other faecal wastes.
3.4 Efficiency of composting in viral inactivation
Few field or laboratory studies are available in the literature on the efficiency of composting for viral inactivation in human faeces. The limited data on the inactivation of bacteriophages in latrine wastes have been described in previous sections of this report. The Danish Environmental Protection Agency has given financial support to projects assessing the survival of different pathogens, including bacteriophages, in composted human faeces. The results from these studies should be available in year 2003.
Due to the absence of data on the fate of human enteric viruses during composting of human faeces, the survival of viruses during composting was reviewed on the basis of studies on composting of faecally contaminated sources, such as sewage sludge, liquid organic waste and domestic solid waste containing disposable diapers. Studies on virus survival during composting of manure or animal carcasses are also presented, because the survival of animal viruses during composting can be expected to be similar to that of taxonomically related human viruses.
3.4.1 Composting of sewage sludge
The efficiency of composting of sewage sludge for viral inactivation has been demonstrated by several laboratory studies and field studies at compost facilities. Early studies on virus inactivation by composting determined the effects of the process on male-specific coliphage f2 as a model virus /110-112/. In both static pile and windrow composting of municipal wastewater sludge, the titer of coliphage f2 decreased over time. The rate of decrease depended on the type of compost system and the season of the year, with more rapid inactivation in warmer seasons. Reductions of 1 log10 were observed in 4 to 7 days in windrows during dry weather. Rainy weather reduced the inactivation rates by about 50%. Reductions were more rapid in aerated piles than in windrows, probably because higher temperatures were achieved.
Kawata et al. /112/ demonstrated that inactivation of seeded coliphage f2 (initial concentration of 106 PFU/g) by a windrow composting system required about 50 days for raw sludge and up to 70 days for digested sludge. A similar study by Burge et al. /111/ showed that coliphage destruction by aerated static piles was achieved deep in the pile within 21 h, although very small concentrations of the phage (about 0.001%) survived at the edge of the pile. Also in the 1960s, two studies reported that seeded poliovirus type 1 was eliminated in sludge composted at 38-58° C for 7 days /113/ and at 60-76° C for 1 hour /114/.
More recent studies have investigated the inactivation of bacteriophages and enteric viruses naturally occurring in raw sludge. Langeland et al. /115/ reported that coliphages M2 in raw sludge (approximately 103 to 104 PFU/g) were completely inactivated after 7 days of composting at 60° C. Carrington et al. /116/ documented that enteroviruses in raw sludge (mean concentration of 25.4 PFU/g) were reduced to below the detection limit (0.1 PFU/g) after 28 days of composting at 55° C.
3.4.2 Composting of liquid organic waste
Composting can also be used for sanitation of liquid organic waste. A composting system for different kinds of liquid biodegradable waste was recently tested in a pilot scale /95/. The bacteriophage Salmonella typhimurium 28B was used as an indicator to evaluate the efficiency of the composting system for viral inactivation in blackwater (faeces, urine, toilet paper and water for flushing) mixed with kitchen organic waste. The results indicated that the die-off was lower at 55° C compared with 60° C. The time required for the inactivation of 90% phages (T90) was calculated to be 14.7 hours at 55° C and 3.5 hours at 60° C.
The authors observed that addition of liquid manure into the waste material reduced the die-off of the bacteriophage (T90 was 23.4 hours at 55° C and 13.7 hours at 60° C) /95/. The reason of the slower die-off rate in waste containing manure was not investigated. However, the results indicate that the composition of the raw material may influence substantially the die-off of viruses during composting.
3.4.3 Composting of animal faeces
Laboratory studies have shown that composting rapidly inactivate viruses in animal faeces. Monteith and Shannon /117/ studied the inactivation of enteric viruses by composting of cattle faeces. Two representatives of the most heat-resistant bovine viruses, i.e. parvovirus and enterovirus, were seeded into the solid fraction of cattle manure at concentrations comparable with the levels found in manure applied in the field (approximately 105 PFU/g). The operating temperatures reported in this study were 30° C on day 1, 45° C on day 2 and 60° C for the rest of the experiment. Results showed that neither the parvovirus nor the enterovirus survived composting for 28 days. Thus, composting appeared to be a suitable method for the disinfection of manure for use as a soil conditioner.
In another study, Hirotani et al. /118/ used porcine faeces as a model to study the fate of viruses during composting. Porcine faeces containing 106 PFU/g of coliphages were kept at 60° C under aerobic conditions. Samples were periodically mixed to maintain aerobic conditions and supplied with water to avoid dryness. No plaques were detected after 5 days, suggesting that more than 99.99% of the coliphages were destroyed under these conditions.
3.4.4 Composting of domestic solid waste
The occurrence of human enteric viruses in domestic solid waste is mainly associated with the presence of faecally soiled diapers, which have been found to contain average concentrations of 146 PFU of polioviruses type 3 in every gram of faeces /119/. Gerba et al. /120/ evaluated the effects of composting domestic solid waste containing several faecally contaminated diapers. No viruses were detected in compost treated by in-vessel composting for 100 to 200 days, with temperatures ranging between 57 and 70° C. Furthermore, the lack of enteroviral detection by DNA hybridization suggested that no intact viral DNA was present in the final compost product.
An investigation performed at a Danish composting plant showed that numbers of coliphages were reduced below the detection limits (2 PFU/100 g) during composting of household waste in open-air windrows /121/. The raw material was found to contain an average of 5´ 103 PFU of coliphages every 100 g.
3.4.5 Composting of poultry carcasses
The use of composting as an alternative to burial, burning and rendering for disposal of poultry carcasses was recently studied /122/. Despite differences in temperature between lower and upper levels of carcasses, two-stage composting (2 cycles of 7 days separated by turning) was found to destroy various avian pathogenic viruses in infected carcasses. The peak temperatures reached during composting were 58.3° C in the upper level and 42.8° C in the lower level /122/.
3.5 Virus persistence in soil
Enteric viruses can persist long after application of contaminated sludge to soil, as indicated by their recovery in sludge burials 6 months after the last sludge disposal /123/. The persistence of certain viral pathogens in soil represents a potential risk for human health. Viruses may be transported for long distances through soil aquifers, with the consequent possible contamination of groundwater reservoirs /124-126/.
High temperature and dry conditions are the two main factors affecting the persistence of viruses in soil /127/. While long virus survival times (over 5 months) are possible under cool conditions, at warmer temperatures (<25° C) viruses are likely to be eliminated within 2 weeks /23/. Under constant moisture conditions, the die-off of enteric viruses are significantly higher at 27° C than at 15° C /128/ and at 23° C than at 10° C /129/. Evaporation to less than 5% soil moisture content completely inactivate viruses within 7 days at 15° C and within 3 days at 27° C /128/. Soil moisture contents below 2.9% have been shown to have a particularly strong virucidal effect /107/
Other factors affecting virus persistence in soil include soil type. This is because soil type influences the extent of virus adsorption to the soil particles, with clay particles being more adsorptive of viruses than sand, silt or organic particles. Virus survival is enhanced by clay soils compared with sandy and organic muck soils, apparently due to greater adsorption and its protective effect /128,130/.Virus type also influences virus survival in soils. The die-off of HAV is lower than for enteroviruses /129,130/, and even enteroviruses differ widely in their persistence in soils and sediments. Furthermore, it appears that soil microorganisms can produce antiviral substances increasing the rate of viral inactivation in soil, as suggested by the increased survival of viruses in sterile soils /131,132/.