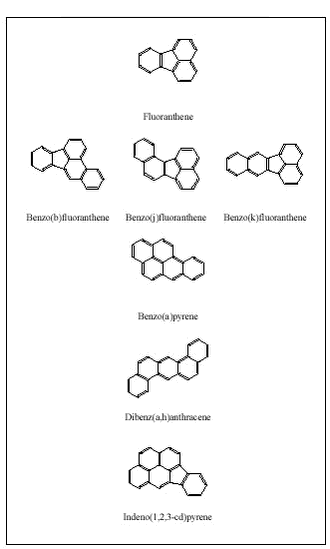
Human Bioaccessibility of Heavy Metals and PAH from Soil4 Soil contaminants4.1 Speciation of PAH in soil4.2 Speciation of metals in soil 4.2.1 Arsenic 4.2.2 Cadmium 4.2.3 Chromium 4.2.4 Copper 4.2.5 Lead 4.2.6 Nickel 4.2.7 Zinc 4.3 Implications of speciation for design of bioaccessibility tests The human uptake is highly dependent upon the chemical conditions encountered during digestion (chapter 3) but also upon the matrix and chemical form (speciation) of the contaminants. The specific physical-chemical properties and potential interactions with soil constituents of each contaminant are controlling the processes of dissolution and transport of the contaminants in the gastrointestinal lumen (i.e.: the bioaccessibility processes). Structures (PAH only) and selected physical-chemical data for the project contaminants:
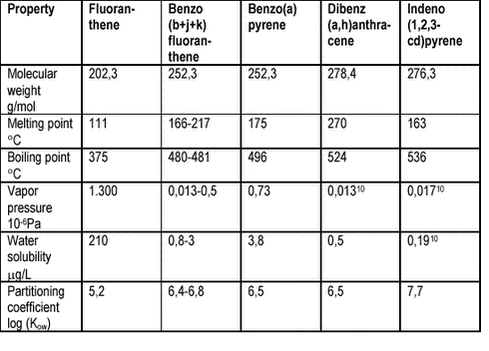
are given in figure 4.1 and in tables 4.1-4.2. Figure 4.1 Table 4.1
The selected PAH are solids at room temperature with high boiling points, low vapor pressures, low water solubilities and high affinity for an organic phase (high log (Kow). Table 4.2
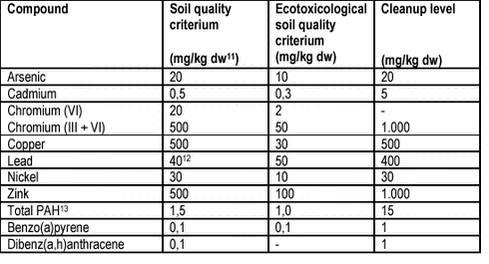
For reference, the Danish soil quality criteria are given in table 4.3. The soil quality criteria are enforced as limits for sensitive use (gardening, day care institutions etc) of the contaminated area, the cleanup levels require intervention, whereas the ecotoxicological soil quality criteria generally are not enforced. For simplicity, these criteria and levels are referred to as MCLs in this review. Table 4.3
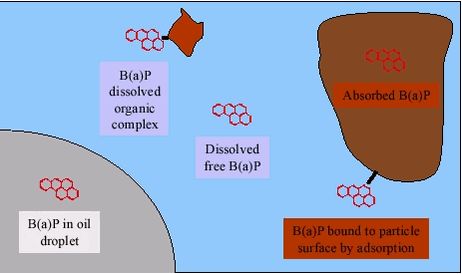
4.1 Speciation of PAH in soilAn example of distribution between phases and chemical forms (species) in soils is shown for benzo(a)pyrene in figure 4.2. Due to their physical-chemical properties, the PAH will primarily be absorbed into the organic matter of the soil, with smaller amounts adsorbed to inorganic soil particle surfaces and adsorbed to dissolved organic matter (dissolved organic “complex”) and a very minor fraction present as free, dissolved PAH. In soils contaminated with separate phases of e.g.: petroleum products, PAH may also be present dissolved in the separate phase. The fraction absorbed into the soil organic matter becomes less desorbable with time, a phenomenon called aging. In very recently contaminated soils, PAH will consequently be more bioaccessible as compared to soils with the same PAH composition and concentration that has aged for years after contamination, even though the PAH are still present. The molecular mechanism behind aging is still debated (e.g.: /37/) and a more detailed discussion is beyond the scope of this review. Still, it should be noted that as low as 10% bioavailability has been measured (as mutagenic activity) for benzo(a)pyrene in soil /38/, suggesting a significant effect of aging. Bioavailability reductions varying from 5% to 50% has been measured (as biodegradation) for 16 different soils /39/, suggesting large differences in the magnitude of the aging effect among different soils. Certainly, it has been suggested /40/ /41/ that the effects of aging should be consider in risk assessment of soils contaminated with compounds that age. The bioaccessibility of the two solid species of benzo(a)pyrene: absorbed, possibly aged in organic matter and adsorbed to mineral surfaces will differ. Likewise, the bioaccessibility of separate phase benzo(a)pyrene will differ from the accessibility of the solid species. The absorption of the two dissolved species: free benzo(a)pyrene and bound to dissolved organic compounds such as humic substances may differ, depending upon the stability of the organic “complex” in the gastrointestinal lumen, see chapter 3. Figure 4.2
The primary mechanism for reduced bioaccessibility of PAH from soil will thus be low solubility and absorption into soil organic matter. The most important factors for release from the soil will be dissolution (“detergent aided” by bile) and release from the soil organic matter. Dissolution of soil organic matter can increase accessibility by increasing the capacity for forming dissolved organic complexes. 4.2 Speciation of metals in soilIn assessing bioaccessibility of metals in soil, three major obstacles are encountered:
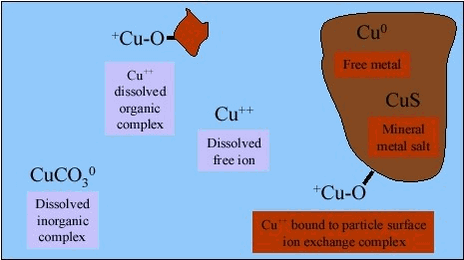
Assessment of bioaccessibility data for metals in soil therefore needs to reflect the varying geochemical conditions. An example of distribution between phases and chemical forms (species) in soils is shown for copper in figure 4.3. The bioaccessibility of the three solid species of copper: free metal (Cu0), copper sulfide (CuS) and copper cations bound by ion exchange mechanisms, will differ. Similarly, the absorption of the three dissolved species of copper: free copper ions, copper ions in inorganic complexes and copper in organic complexes with e.g.: humic substances or organic acids, may differ, depending upon the stability of the complexes in the gastrointestinal lumen, see chapter 3. Figure 4.3
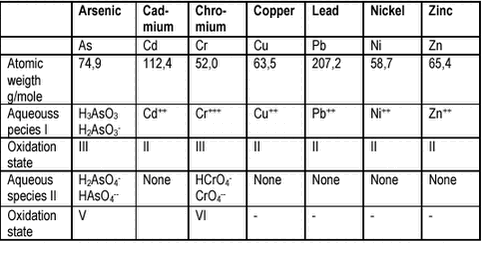
An aging effect (compare section 4.1) in soils has been observed for As(V) /42/ and Cr /43/, see below and chapter 7. It is important to remember that some heavy metal bearing minerals have resisted weathering and dissolution over geological time scales. Whether the aggressive chemical conditions in the human digestive tract nevertheless will cause dissolution, depends upon the mineral. Due to their different physical-chemical properties, the mechanisms for reduced bioaccessibility of metals differ among the metals. Summaries of relevant physical-chemical properties are compiled below from review papers and textbooks on metals /3;5-7;9;13;35/. The summaries emphasise the chemical conditions relevant to soils and to the human digestion only, e.g.: pH between 1 and 8. 4.2.1 ArsenicIn fully oxidised water such as most soil pore waters, the primary aqueous species will be arsenates (oxidation state V), and in reduced waters arsenites (oxidation state III), table 4.2. For the arsenates, the mono-anionic dihydrogen arsenate will dominate at low pH (below pH 6,9), and for the arsenites, the non-ionic trihydrogen arsenite will dominate below pH 9,2. Arsenic salts with low solubility include calcium, iron and manganese arsenates. The direct effect of pH on water solubility of arsenic salts will be limited. Arsenic is found in all rocks and soils at low concentrations (typical 2 mg/kg, median from Danish soil quality monitoring: 3,3 mg/kg dw /44/) and in a variety of species, with arsenopyrite (FeAsS) as the most common. Frequent anthropogenic (Man made) sources are metal mining and smelting (arsenic trioxide, As2O3), and tanneries, pesticides and wood preservatives (arsenate, H2AsO4-). In soils, arsenic may be found associated with iron sulphides and iron oxyhydroxides, and arsenate – iron oxide associations are more stable than for arsenite /45/. Organic acids and other anions such as phosphates compete with arsenate and arsenite for iron oxyhydroxide sites /45;46/.Organic arsenic may be formed in soils. For As(V), aging has been observed and the effect can be explained by formation of mixed minerals with iron oxyhydroxides (inner sphere surface complexes) at low pH (pH < 6) within a period of less than 3 months (see also chapter 7) /42/. Overall, the primary mechanism for reduced availability of arsenic in soil will be minerals of low solubility and adsorption to iron oxyhydroxides, and the most important factor for release from the soil will be (acidic) dissolution of the arsenic minerals and the iron oxyhydroxides. Reduction of arsenate to less efficiently sorbed arsenite and displacement by other anions such as organic acids and phosphate may be important release mechanisms as well. 4.2.2 CadmiumCadmium occurs in natural waters in oxidation state +II as the cation Cd++ and complexes of Cd++: cadmium hydroxide (CdOH+) and carbonate (CdCO30) at high pH; cadmium sulphate (CdSO40) and chloride (CdCl+) at lower pH and depending upon the cadmium concentration. Cadmium salts with low solubility includes cadmium phosphate (Cd3(PO4)2), sulphide (CdS), hydroxide (Cd(OH)2) and carbonate (CdCO3). The solubilities of these cadmium salts will increase with decreasing pH. Cadmium is found in all soils at low concentrations (typical 0,1-0,4 mg/kg, median from Danish soil quality monitoring: 0,16 mg/kg dw /44/) and in a variety of species, mainly as sulphide and carbonate, but also as phosphate. Frequent anthropogenic sources are metal (zinc) mining and smelting due to co-occurrence of cadmium sulphide with the important zinc ore: zinc sulphide. Phosphate fertilisers, atmospheric deposition and sewage sludges are other important sources of cadmium in soils. Metal plating industries, PVC stabilisers, batteries and pigments are potential cadmium sources. In soils, cadmium will also be found bound to the cation exchange sites (clay minerals, iron oxyhydroxides and calcium carbonate minerals). Overall, the primary mechanism for reduced availability of cadmium in soil will be minerals and salts of low solubility and cation exchange, and the most important factor for release will be (acidic) exclusion from and/or dissolution of the cation exchange complexes, as well as acidic dissolution of cadmium minerals and salts. 4.2.3 ChromiumIn fully oxidised water such as most pore waters, the stable aqueous species will be chromates (oxidation state VI), and in reduced waters chromium cations (oxidation state III). For the chromates, the mono-anionic hydrogen chromate will dominate below pH 6,5, and for chromium cations, complexes of the trivalent cation with hydroxide will dominate above pH 4. The conversion between the two oxidation states depend upon the presence of catalysts, the redox conditions and the pH and both oxidation states can thus be present in soils depending on these environmental factors and on the oxidation state of the chromium source, see also chapter 7. The extent of conversion between the two redox states (“chromium cycling”) is disputed and for practical purposes, Cr(III) can be considered stable, whereas Cr(VI) can convert slowly to Cr(III) in natural systems. Chromium salts with limited solubility include hydroxides of Cr(III) and calcium, lead, zinc and barium salts of Cr(VI). The solubility of Cr(III) hydroxides will increase with decreasing pH. Chromium is found in all soils at varying concentrations (typical 10-50 mg/kg, median from Danish soil quality monitoring: 9,9 mg/kg dw /44/) depending upon the soil parent material and from natural sources mainly as Cr(III), whereas occurrence of Cr(VI) is almost exclusively the result of human activities. Frequent anthropogenic sources include mining and smelting (primarily chromite, FeO Cr2O3), metal plating and corrosion control, wood treatment, leather tanning and pigments. In soils, Cr(III) will mainly be found as precipitated hydroxides and possibly bound to the cation exchange sites (clay minerals and iron oxyhydroxides), whereas Cr(VI) will be found bound to iron oxyhydroxides. For Cr(VI), an aging effect has been observed and can be explained by conversion of more soluble Cr(VI) to Cr(III) cations that are bound to the soil in cation exchange complexes or as hydroxides (see also chapter 7) /43/. For Cr(III), an aging effect has been observed that can be explained by slow (50 days) transformation of comparatively bioaccessible Cr+++ bound in particle surface ion exchange complexes to less bioaccessible Cr(OH)3. Overall, Cr(III) will have reduced bioavailability in soils as the low solubility salt Cr(OH)3, whereas binding to iron oxyhydroxides to some degree will be a factor for Cr(VI). The most important factor for release will be (acidic) dissolution of Cr(OH)3. 4.2.4 CopperIn natural waters, the most important Cu species will be Cu++ (in hydrated form), the hydroxy complexes (e.g.: CuOH+ and Cu(OH)20) and the carbonate complexes (CuCO30 and CuHCO3+). Organic complexes will also be present. Copper salts with limited solubilities include hydroxides, carbonates and sulphides. Particularly insoluble is copper hydroxide (Cu(OH)2) and overall, copper has a very limited solubility at pH above 7-8. The solubility of copper hydroxide will increase with decreasing pH. Copper is found in all soils at varying concentrations (typical 30 mg/kg, median from Danish soil quality monitoring: 0,9 mg/kg dw /44/). The natural occurrences are dominated by copper sulphides (including mixed sulphides with iron) and hydroxycarbonates (e.g.: malachit CuCO3 Cu(OH)2). Frequent anthropogenic sources include waste incineration slags (tenerite, CuO), manure from live stock treated with growth promoters, wood preservatives, metal industry and electronical industry. In soils, copper will primarily be found as hydroxides, carbonates and sulphides, and also as bound to the cation exchange sites of soil organic matter and iron oxyhydroxides. Metallic copper (Cu0) is a frequent soil contaminant. Overall, reduced bioavailability of copper in soils can be attributed to occurrence of metallic copper, low solubility salts and cation exchange complexes. The most important factors for release will be (acidic) dissolution of carbonates and hydroxides, (acidic) exclusion from or dissolution of iron oxyhydroxide complexes and (alkaline) dissolution of organic complexes. 4.2.5 LeadIn natural waters, the most important Pb species will be Pb++ and the carbonate complex PbCO30. Sulphate, chloride and organic complexes will also be present. At low pH, PbSO40 will dominate and at high pH, PbCO30. Most lead salts are insoluble with lead nitrate and to some degree lead chloride as the important exceptions. Lead phosphates (e.g.: Pb3(PO4)2 and PbHPO4) as well as mixed lead chloride phosphate (Pb5(PO4)3Cl) are very sparingly soluble, but lead sulphate, carbonate, hydroxide and sulphide exhibit limited solubility as well. Lead also forms insoluble salts with some organic acids. Lead carbonate and hydroxide typically found in alkaline soils will exhibit increasing solubility with decreasing pH, whereas the lead sulphate and phosphates found in acidic soils will respond less to acidification. Lead is found in all soils at varying concentrations (typical 5-30 mg/kg, median from Danish soil quality monitoring: 11 mg/kg dw /44/). The natural occurrences are dominated by lead sulphate, sulphide and carbonate. Frequent anthropogenic sources include metal mining, smelting and processing, traffic (leaded gasoline), incineration processes, disposed lead acid batteries (in particular metal scrap sites), paints and waste. Although organic lead compounds are released from traffic and with gasoline spills, their transformation to inorganic lead compounds are believed to be fast and their significance in soils therefore low. In soils, lead phosphates dominate, followed by carbonates and hydroxides. Complex formation with cation exchange sites may be important, in particular with iron oxyhydroxides and organic matter. Lead is considered the least mobile of the heavy metals in soils. Overall, reduced bioavailability of lead in soils can be attributed to occurrence of low solubility salts and maybe to cation exchange complexes. The most important factor for release will be (acidic) dissolution of lead carbonate and hydroxide, but phosphates present will counteract release. 4.2.6 NickelIn natural waters, the most important nickel species will be the Ni++ cation and complexes with carbonate (NiCO30) and with organic compounds. Hydrogencarbonat complexes (NiHCO3+) complexes and at high pH also hydroxy complexes may be present. Nickel salts with limited solubility includes nickel phosphate (Ni3(PO4)2), nickel hydroxide (Ni(OH)2) and sulphide (NiS) and to some degree nickel carbonate (NiCO3) , whereas most other nickel salts are soluble in water. The nickel salts are more water soluble than most of the other cationic heavy metals. Solubilities of these salts will increase with decreasing pH. Nickel is found in all soils at varying concentrations (typical 5-15 mg/kg, median from Danish soil quality monitoring: 4,0 mg/kg dw /44/). The natural occurrences are mostly mixed ore sulphides with iron or copper, but nickel is also present in elevated concentrations in pyrite (FeS2). Frequent anthropogenic sources include mining and melting, metal processing and nickel plating, as well as nickel-cadmium batteries. In soils, nickel bound to cation exchange sites in organic matter, iron oxyhydroxides and clay minerals will dominate. Overall, reduced bioavailability will mainly be caused by cation exchange but the presence of low solubility salts may be of importance for alkaline soils. Release will primarily be (acidic) exclusion from and dissolution of ion exchange complexes. 4.2.7 ZincIn natural waters, zinc will mainly be present as the cation Zn++ but complexes with carbonates (ZnCO30 and ZnHCO3+) and hydroxide (e.g.: ZnOH+) may be present as well. The importance of organic complexes is not well described. Zinc salts with limited solubility include zinc phosphate (Zn3(PO4)2), zinc sulphide (ZnS), zinc hydroxide (Zn(OH)2) and zinc carbonate (ZnCO3). Zinc phosphate, hydroxide and carbonate will exhibit increasing solubilities with decreasing pH. Zinc is found in all soils at varying concentrations (typical 10-300 mg/kg, median from Danish soil quality monitoring: 19 mg/kg dw /44/). The natural occurrences are mainly zinc sulfide (ZnS). Frequent anthropogenic sources include mining and smelting, metal processing and plating, fertilisers and sludges. In soils, zinc will primarily be present bound to cation exchange sites of iron oxyhydroxides, clay minerals and organic matter. Reduced bioavailability will mainly be by ion exchange and to some degree by the presence of low solubility salts for alkaline soils. Release will be by (acidic) exclusion from and dissolution of ion exchange complexes. 4.3 Implications of speciation for design of bioaccessibility testsA test for bioaccessibility of contaminants in soil should be designed to simulate a realistic worst case scenario, i.e.: it should enable estimation of the highest bioaccessibility likely to occur. Consequently, test design should include:
_____________________________________________________________________ |