Human Bioaccessibility of Heavy Metals and PAH from Soil
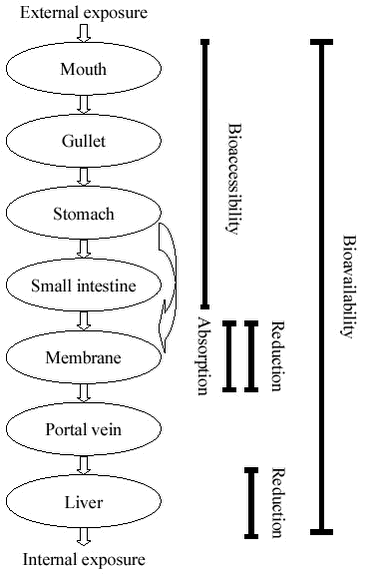
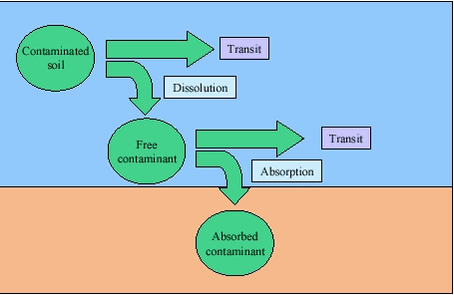
3 Physiology of human contaminant uptake3.1 Implications of human contaminant uptake physiology for design of bioaccessibility testsA series of compartments are involved in human uptake of ingested soil contaminants, figure 3.1. Figure 3.1
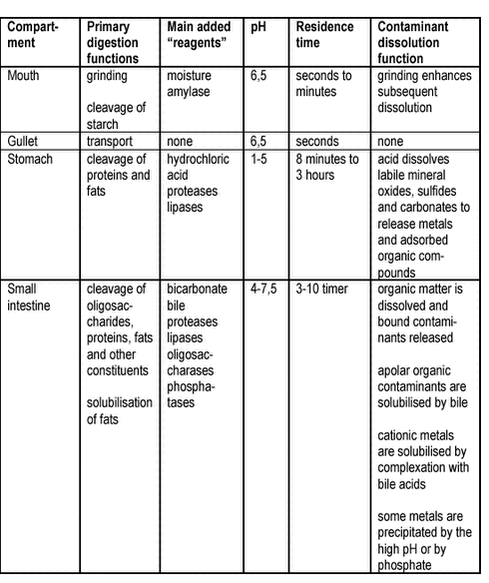
The overall pathway leads the food and soil with contaminants from the mechanical grinding in the mouth through a series of chemical and microbiological processes to partial dissolution through the entire gastrointestinal tract (bioaccessibility processes). The dissolved components are transported through the membranes of the gastrointestinal epithelium (absorption) and into the blood stream. During transport through the membranes, degradation can occur (reduction). The blood passes the liver before entering the systemic circulation allowing for degradation or removal of unwanted compounds in the liver (reduction, first pass effect). It should be noted that in medical and toxicological literature, the term absorption often pertains to absorption into the systemic blood stream, i.e.: absorption includes the process of first-pass metabolism. Most of the dissolution processes are completed before the material is leaving the small intestine, and it is generally accepted that most of the uptake takes place in the small intestine /25/. To which extent uptake takes place in the stomach is currently not clear. The environment in the compartments differs and accordingly impacts the bioaccessibility process differently, table 3.1. Table 3.1
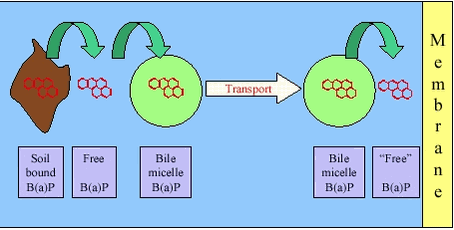
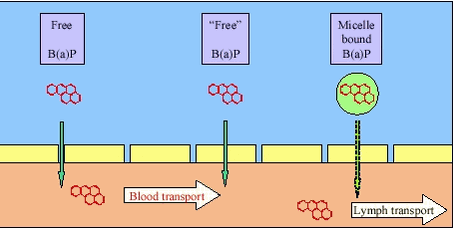
It should be noted, that the gastrointestinal tract constitute a complex ecosystem with aerobic and anaerobic microorganisms /26/. The density of microrganisms is less in the human stomach and in the upper part of the small intestine but increasing towards and in the large intestine. In human faeces, anaerobic microorganisms dominate, whereas aerobic bacteria are found in high densities higher in the large intestine /27/. Sulphate reducing bacteria have been detected in the human large intestine /28/ but on the other hand, high concentrations of oxygen have been detected throughout the gastrointestinal tract of pigs /29/. Overall, dominating aerobic conditions and microorganisms would be expected in the stomach, but with increasingly anaerobic conditions from the small intestine to the large intestine. Absorption requires that the contaminants are dissolved (free or bound to a dissolved carrier such as bile), transported to the gastrointestinal wall and, if bound to a carrier, released at the surface of the gastrointestinal membrane for absorption, figure 3.2. The carrier mechanisms can be dissolution of apolar contaminants in bile micelles or complexation of cationic metals by bile acids. For apolar contaminants such as many PAH, the carrier will counteract the low water solubility and thus enhance exposure of the membrane to freely dissolved contaminants. Likewise, bile acids, proteins and other complexing agents can enhance exposure for cationic metals. Also, lipids and other soluble organic matter in the diet can add to the carrier effect of the bile. Figure 3.2
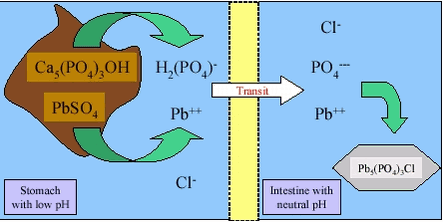
Unfortunately, the simple dissolution – transport –absorption processes can be complicated by the sequential change in the chemical environment of the gastrointestinal tract, as well as by soil and contaminant chemistry. As an example, lead found in soil as the common contaminant anglesite (PbSO4) will dissolve in the stomach and will stay in solution at the low pH and high chloride concentration here, figure 3.3. Figure 3.3 Entering the higher pH in the presence of dissolved phosphate in the small intestine, the dissolved lead ions (Pb++) will precipitate very quickly as chloroleadphosphate (chloropyromorphite, Pb5(PO4)3Cl) /30/. The phosphate can originate from digested food or from the soil. Phosphate minerals, such as hydroxyapatite, Ca5(PO4)3OH, will dissolve in the low pH of the stomach, but dissolution will be slower and less complete at higher pH in the stomach (as occurring after food ingestion). If stomach transit is fast (as occurring under fasting conditions), the hydroxyapatite may not dissolve in the stomach and reach the small intestine where the neutral to slightly alkaline pH will prevent further dissolution and thus also precipitation of released lead as chloroleadphosphate. Conversely, just after transit from the stomach to the small intestine, the pH is still low and absorption of lead can take place driven by the high dissolved lead concentration possible in acidic pH. Overall, the de facto dissolution of lead from soil will depend upon interacting conditions such as soil composition, simultaneously ingested food and feeding condition of the human. The absorption of dissolved contaminants is through the epithelium of the stomach and the small intestine (the intestinal epithelium) either through the cells (transcellular transport) or between the cells (paracellular transport), figure 3.4. The pathway through the cells is primarily taken by apolar contaminants (e.g.: PAH) that can easily pass the lipid phase of the membranes, whereas the pathway between the cells is primarily taken by polar or ionic contaminants (e.g.: some metals). The transport of apolar organic contaminants through the cells is by passive diffusion. Active transport across the membrane requires that the contaminant “fits” into a transport system already present (e.g.: the monosaccharide transport system) and this has not been demonstrated for PAH. In addition, it has been suggested /2/ that absorption of apolar contaminants can occur by the fatty acid route with the contaminants entering the organism through the lymph system and not through the blood stream. In principle, this pathway is based upon transport of micelles of lipids, bile and contaminants towards the membrane, diffusion across the cell membrane, re-incorporation of the contaminants in mixed micelles with lipids followed by secretion of these into the lymphatic circulation /31/. This pathway has not been supported for PAH. Figure 3.4
Metals are absorbed by passive paracellular transport, by passive, transcellular diffusion or by active, transcellular transport fitting into a transport system already present. One example is that cadmium can be absorbed by both the passive paracellular route and the passive diffusive route /32/. Another example is lead, that is probably absorbed via the calcium uptake system(s) including both active and passive transcellular transport, as well as by paracellular transport /33/. Reduction and transformation of the absorbed contaminant concentrations takes place in the epithelium membranes (binding and exclusion) and cells (degradation and transformation of organic contaminants), as well as in the liver (degradation and transformation of organic contaminants, transformation of metals, and secretion of metals and PAH with bile). Contaminants entering systemic circulation via the lymph will be less efficiently reduced, as the liver is bypassed for this route. Finally, the contaminants are diluted when entering systemic circulation in the blood stream. If we consider the sensitivity of the processes of dissolution, absorption and reduction to changes caused by varying “vehicles” (i.e.: ingestion with soil, food or in solution) and chemical forms (i.e.: different metal salts ingested), we would expect dissolution to be highly sensitive, absorption to be sensitive and reduction to be slightly sensitive (chemical form) or insensitive (vehicle). In applying the concept of relative bioavailability (chapter 2), the most important factor to assess would thus be the bioaccessibility factor fb (figure 2.1) followed by the absorbability factor fa. Estimation of the relative bioavailability factor thus reduces to an estimation of how the two potentially rate limiting processes of dissolution and absorption responds to variations in vehicle and chemical form of the contaminants, figure 3.5. If the dissolution process is rate limiting (i.e.: if dissolution is slower than absorption), changes in fb will determine the relative bioavailability. If the absorption process is rate limiting (i.e.: absorption of dissolved contaminants is to slow to be completed before transit), fa will be “in charge” of relative bioavailability, see also chapter 8 for elaboration of the relationship between bioaccessibility and bioavailability in the soil contaminant context. Figure 3.5
3.1 Implications of human contaminant uptake physiology for design of bioaccessibility testsA test for bioaccessibility of contaminants in soil should be designed to simulate a realistic worst case scenario based upon the description of the human digestion and uptake processes, i.e.: it should enable estimation of the highest bioaccessibility likely to occur. Consequently, test design should include:
|