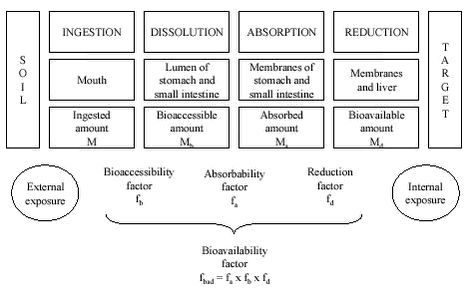
Human Bioaccessibility of Heavy Metals and PAH from Soil2 Human risk assessment for soil contaminantsThe highest concentrations of contaminants that are acceptable in soils are generally based upon estimates of human exposure (how large amounts of the contaminant can impact the human via the sum of exposure routes) and of the toxicity of the contaminants to humans. Thus quality criteria for soil (the maximum contration limits for soil) are calculated on the basis of a tolerable daily intake value (TDI) or a provisional, tolerable weekly intake (PTWI), that can be derived from the no observed adverse effect level (the NOAEL) found in human data or experimental animal data. For genotoxic carcinogens for which no lower threshold for increased risk for cancer risk is assumed, the TDI value is set at a level that corresponds to a tolerable low (negligible) cancer risk level. In Denmark, the TDI is set to a dose comparable to an excessive risk of 10-6 i.e.: a calculated hypothetical risk of one extra cancer outcome among 1 million people in a lifetime. In calculating the tolerable soil exposure estimates, the impact of other sources is taken into account by allocating the total tolerable amount to different exposure routes, e.g.: food, drinking water and soil. The allocation is given as the allocation factor (fa) which is the fraction of TDI that is allowed from soil exposure. Oral ingestion is one of the most important exposure routes for humans to soil contaminants /18/, and the Danish MCLs are in most cases developed based upon oral uptake by children /19/. The MCL for soil ingestion is obtained by dividing the TDI (corrected for allocation) with the estimated daily soil exposure (EDE). MCL (mg contaminant/kg soil) = TDI (mg contaminant/person/day) x fa/EDE (kg soil/person/day) For determining the TDI, data on oral toxicity are primarily considered. Often these data pertain to animal experiments where the substance is administrated to the animals mixed in the feed or in drinking water (the vehicle or transporter of the contaminant). As an alternative, epidemiological studies relating observed human health effects to recorded exposures have been used1. The amount of contaminant needed to produce adverse health effects in the animal is then recorded. Most toxicologicalstudies report the total ingested amount only and do seldom indicate exact values for the bioavailability of the substances administered. When extrapolating from such experimental conditions to other conditions e.g.: to intake of contaminated soil, this approach requires, that the uptake efficiency is equal for all scenarios, i.e.: that the absolute bioavailability, AB, of the contaminant is constant. The absolute, oral bioavailability can be defined as: AB = internal dose/external (administered) dose In words, the absolute, oral bioavailability is the fraction of an orally ingested contaminant that reaches systemic circulation, i.e.: enters the blood stream. The absolute oral bioavailability of a contaminant may range from close to 0 to almost 1 (i.e.: 100%) depending upon the physiochemical form of the contaminant. In this context, the use of the concept of absolute, oral bioavailability rests upon the assumption that adverse health effects are systemic and thus triggered by the contaminants reaching the blood stream, i.e.: the internal exposure as opposed to the external exposure measured directly as intake of contaminated medium multiplied by the concentration of the contaminant in the medium, figure 2.1. The absolute bioavailability can be measured as the ratio between amounts in the blood of laboratory animals after intravenous injection (100% uptake) and after oral ingestion (uptake of bioavailable fraction). Alternative and less direct approaches are at hand as e.g.: measuring the absorbed amount of orally ingested contaminant as the amount that is excreted with urine, see chapter 5. Figure 2.1
A more feasible approach is to measure the relative bioavailability or relative absorption fraction (RAF). RAF is obtained as: RAF = amount taken up from new matrix/amount taken up from the matrix used in the toxicity study In words, the relative bioavailability is the ratio between the amount of a contaminant reaching systemic circulation when ingested with e.g.: soil and the same amount obtained when ingested in the toxicity experiment. If the relative bioavailability of a contaminant deviates from 1 (~100%) when ingested in soil as compared to ingestion in the toxicity experiments behind the TDI, a correction of the MCL to account for this can be argued for. If a reliable and safe RAF value can be found and agreed upon, this would then result in a proportional change in the MCL: MCLtrue = MCL/RAF For substances where the critical toxic effect is not systemic toxicity but local toxicity (i.e.: local irritation), the toxic effect is considered to be dependent of the concentration in the gastrointestinal tract, and the MCL will be dependent of bioaccessibility rather than the bioavailability. It should be noted that although most relative bioavailabilities are less than 1 and would result in an increased MCL, RAF values above 1 could be found that would result in a demand for a decreased MCL. In the US and Canada, the RAF values have been used to increase cleanup levels after risk assessment on a case by case basis, see chapter 9 for examples. In summary: adjustment of cleanup levels based upon bioavailability studies has been reported from the US for arsenic, lead, mercury, PAH, PCB’s and dioxins /8;9/ and from Canada for lead and nickel /20/. In Denmark, specific considerations regarding bioavailability have only been made for few substances, e.g.: nickel, when calculating the MCL. The US EPA allows for using the concept of relative bioavailability in risk assessment /21/, but does not give guidance to the practical implementation, see chapter 9. According to the recent reviews /8;9/, several state regulatory agencies have issued guidance documents. Adjustment of the bioavailability is an option in the US EPA model for risk assessment of lead uptake in children /22/. In vivo data for relative bioavailability are in most cases required to allow the adjustment of lead bioavailability. This reflects the general attitude in the US EPA: that bioavailability based adjustments of maximum contaminant levels or cleanup levels should be based upon in vivo studies with experimental animals resembling humans, e.g.: with immature swine /20/. Still, the US EPA is moving towards accepting “validated” in vitro tests for lead and is chairing a meeting on this subject in April 2003. As set of general factors to be considered deciding whether to include bioavailability studies at a site has been suggested /4/: + Limited number of critical contaminants Table 2.1
In Europe, the emphasis in risk assessment is to develop in vitro tests for bioavailability of soil contaminants /14/. The rationale behind this is that in vitro tests:
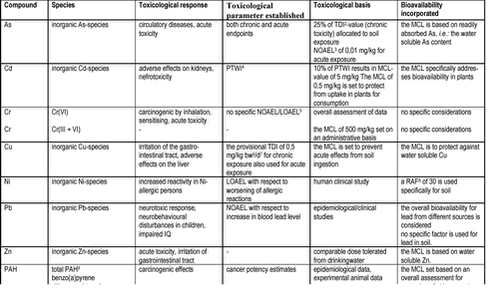
In Denmark, bioavailability of soil contaminants is currently not part of the risk assessment at contaminated sites /1;23/ and has only been addressed in the MCL setting to the degree allowed for by the limited data available /19;24/. A summary of the toxicological data behind the Danish soil MCL values is given in table 2.1, and the Danish MCLs are summarised in table 4.3. |