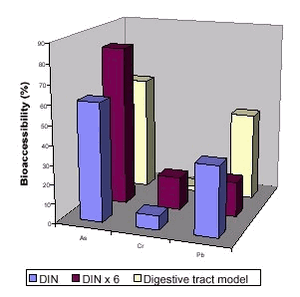Human Bioaccessibility of Heavy Metals and PAH from Soil6 Quantification of bioaccessibility6.1 Chemical extraction tests6.2 Digestion tests 6.2.1 Product methods 6.2.2 Methods for pharmaceuticals 6.2.3 Contaminated soil methods 6.2.4 Comparisons of contaminated soil methods 6.3 Bioaccessibility test performance requirements 6.4 Implications of method studies for bioaccessibility testing A test for bioaccessibility of soil contaminants in soil must enable quantification of the dissolution under “realistic worst case conditions”, meaning that the test should simulate the highest bioaccessibility that can be expected without including unrealistic conditions or excessive precaution. To fulfil this, test must be based upon the properties of the human digestion process, of the contaminants in question and the geochemistry of soils, see chapters 3 and 4. If the test shall be used for practical risk assessment, it furthermore needs to fulfil the common, basic requirements for a regulatory method. The method must be:
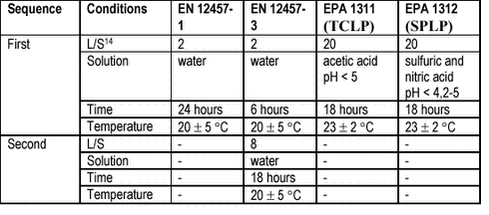
The in vitro bioaccessibility tests range from very simple chemical extraction or leaching tests to advanced multistep tests simulating in detail the human digestion processes. 6.1 Chemical extraction testsSimple chemical extraction tests are used to evaluate the leaching risks associated with solid wastes and contaminated soils /48/. Examples of leaching tests are the European Norm EN 12457 and the US EPA methods 1311 (TCLP) and 1312 (SPLP) /49-51/, see table 6.1 for principles. The toxicity characteristic leaching procedure (TCLP) is designed to simulate the leaching by slightly acidic organic acids in a waste deposit, whereas the synthetic precipitation leaching procedure (SPLC) is intended to simulate leaching by slightly acidic precipitation. Evidently, the leaching tests do not fulfil the requirements set up for bioaccessibility tests. Accordingly, lack of correspondence has been reported for lead in soils between TCLP testing and bioaccessibility testing /9;52/, see figure 6.1. Testing for bioaccessibility with the simple stomach simulating test GJST, see table 6.3, evidently released a higher fraction of soil lead than the TCLP leaching test. Furthermore, the ratios between the dissolution data for the two tests were varying for different soils and even for different soil particle fractions within the same soil, indicating qualitative differences (i.e.: different mechanisms simulated) in addition to the expected quantitative differences (i.e.: different yields of dissolution). The same pattern was reported for Cu and Zn /53/. Table 6.1
Figure 6.1
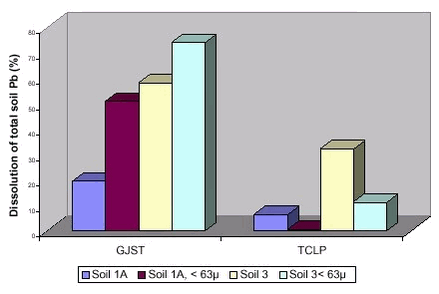
Data from a preliminary comparison (52 soils, 11 metals) of simple chemical extraction (dilute nitric acid, pH = 1,07) with bioaccessibility testing according to the RIVM method (see table 6.3)(unpublished DHI data) demonstrates the lack of correspondence between the two test types for zinc, copper and arsenic, figure 6.2. For zinc, the bioaccessible14 fraction was lower than the extractable, for arsenic higher and for copper comparable. Furthermore, the ratios between extractable and bioaccessible fractions were not the same for the two soils. Overall, simple chemical extraction can not at present be recommended as a test for bioaccessibility of soil contaminants. Figure 6.2
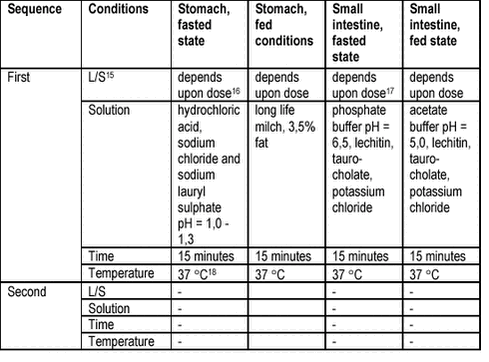
Sequential chemical extraction schemes (e.g.: /54/) are used for speciation of metals in soils or waste and also for evaluation of potential leaching to the groundwater. From the dissolution profile obtained after treatment with reagents of increasing solubilising effect, metal such as arsenic can be characterised as surface sorbed, bound in iron oxyhydroxides or bound in insoluble salts /55/. An attempt to correlate lead, copper and zinc sequentially extracted fractions in mine waste contaminated soils with bioaccessibility measured with the PBET method (see table 6.3) did not succeed /56/. Similarly to the sequential extraction schemes used for metals, methods have been suggested for partial extraction of organic contaminants as an estimate of bioavailability or bioaccessibility (e.g.: /57-59/. The methods are based upon partial extraction with an organic solvent or an extraction method that can dissolve only those contaminants that are not firmly bound in the soil. The partial extraction can then be correlated to bioaccessibility or bioavailability and used as a fast surrogate for bioavailability or bioaccessibility testing. The approach has mostly been used as a test for bioavailability of organic contaminants to the soil biota, but correlation to human bioaccessibility has been attempted for pesticides from soil without convincing results /59/. The interpretation of sequential dissolution tests in terms of metal speciation is debated and the use of partial extraction for organic contaminants is not ready for routine use, in spite of the first promising results with combinations of aqueous and organic solvent extractions /60/. For both metals and organic contaminants, the sequential or partial chemical extraction methods do not satisfy the requirements stated for bioaccessibility methods and are therefore not recommended for this purpose. Still, sequential or partial extraction methods may be of use in evaluating whether a contaminated soil is a candidate for risk assessment based upon bioavailability, i.e.: by answering the question, is the form of the contaminant likely to exhibit low RAF in soil, see chapter 2. 6.2 Digestion testsBioaccessibility tests that simulate the processes in the human gastrointestinal system, the digestion, have been developed for use in studies of drug uptake in pharmaceutical studies, of metal uptake in nutritional studies and of contaminant release from toys. In risk assessment of contaminated soils, digestion tests have been developed since the early 1990’s. 6.2.1 Product methodsRelease of metals from art materials, e.g.: toys, textiles and paints, can according to be tested by simple extraction with pH = 1,2 hydrochloric acid at liquid to solid ratio, L/S = 50 and 37 ° C for 1 hour /61/. The method resembles the European Standard for Safety of Toys, Migration of Certain Elements /62/. In addition to the simple extraction with hydrochloric acid, the European Standard allows for additional extraction with an organic solvent. The methods are intended to give an estimate of the bioavailability of metals after ingestion. It has recently been suggested to include extraction simulating the conditions in the human gastrointestinal system in order to improve the correspondence with in vivo bioavailability data /63;64/. Product methods are currently not satisfying the requirements stated for soil contaminant bioaccessibility test methods. 6.2.2 Methods for pharmaceuticalsDissolution tests form an integrated part of the development of drugs. The standard method is described in the U.S. Pharmacopeia (USP) and involves one step dissolution from a rotating container (paddle or basket) in simple media adapted to the drug and drug formulation in question/65;66/. Several more advanced dissolution tests simulating the processes in the gastrointestinal system have been proposed, e.g.: /25;34/, see table 6.2. The test principles are acidic dissolution with added synthetic tenside (enhances wettability and prevents adsorption to equipment surfaces) simulating dissolution in the empty (fasted state) stomach and long life milk simulating stomach dissolution in the fed state. Neutral dissolution with added synthetic chyme (bile salts and phospholipids) are used to simulate dissolution in the upper parts of the small intestine with slightly lower pH and higher buffer and bile concentrations for the fed state. For lipophilic drugs (compare PAH), it has been clearly demonstrated that the use of high buffer/high bile concentrations simulating fed state dissolution in the small intestine results in higher dissolution of the compounds /34/. For drugs formulated in lipid solutions or emulsions (compare PAH in separate oil phase), an advanced test with addition of lipases (enzymes hydrolysing lipids) and continuous titration (pH stat) maintaining constant pH has been suggested /67/. Currently, a diversity of methods (e.g.: varying pH, buffer and bile concentrations simulating fasting and fed conditions differently) has been proposed as illustrated in a recent review presenting 9 different methods all intending to simulate drug dissolution in the small intestine /67/. It should emphasised that the purpose of drug dissolution tests is to verify sufficient drug dissolution (e.g.: 85%) in the correct compartment (stomach, small intestine or large intestine). Furthermore, drug dissolution tests are generally used in development of drugs and drug formulations and followed by in vivo tests of bioavailability. An alternative use of the dissolution tests is in quality control during production. Therefore, drug dissolution tests with a specified method and with a specified, good precision are required to produce statements like:
Table 6.2
Drug dissolution tests generally are done with the formulation in question, i.e.: without disturbing matrices such as soil. Among the lessons learned from drug dissolution experiments with bearings for bioaccessibility tests are /25;34;68/:
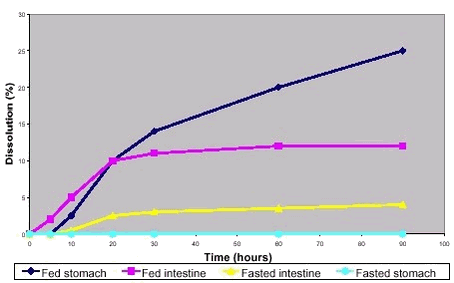
The typical data form for a drug dissolution test is presented schematically in figure 6.3. The data interpretation would be that the tested drug was poorly water soluble, not dissolved in the stomach in the absence of food, dissolved to some degree in the small intestine but reached a solubility limitation here, probably caused by insufficient concentration of solubilising bile constituents. Figure 6.3
Since 1997, the US Food and Drug Administration (US FDA) has endorsed use of in vitro dissolution tests as a surrogate for in vivo uptake investigations in bioequivalence studies (i.e.: testing whether two products have similar uptake properties) /70/. A guideline for establishing in vitro in vivo correlations (IVIVC) has been released for drugs where dissolution is the limiting step for uptake. The approach includes:
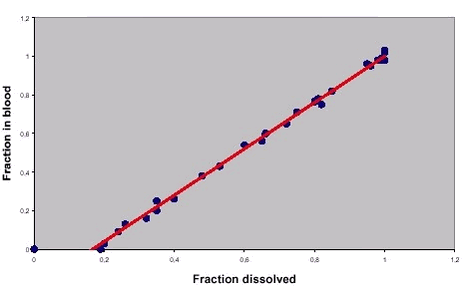
In the hypothetical example of a linear relationship presented in figure 6.4, please note that that the relationship is not a simple 1:1 relationship, i.e.: the line is not through (0,0) with slope 1. In other words, if 50% of a drug is dissolved in the test, the resulting total uptake might be 20%. It should also be noted, that the actual IVIVC depends upon the details of the employed test method, e.g.: fasted or fed state simulation of small intestine dissolution /71/. The logical next step in development of dissolution tests for predicting resulting in vivo blood concentrations is the use of models to predict uptake directly from dissolution tests data and thus circumventing the need for in vivo uptake studies. This development has started (e.g.: /72/) but evidently, the modelling tools have not yet been developed to yield satisfying predictions. Figure 6.4
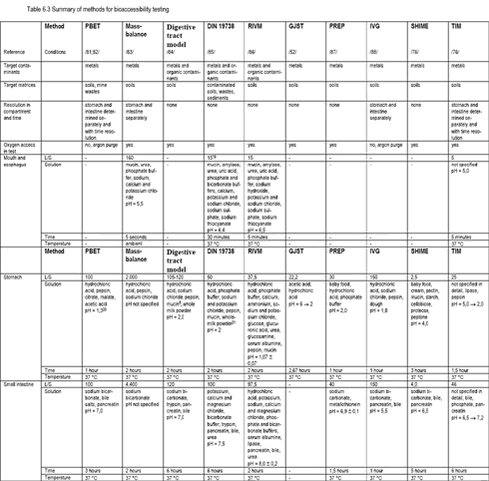
In routine pharmaceutical applications, the different dissolution tests for different uptake compartments are used separately, e.g.: one test for fed state small intestine dissolution, one test for fed state stomach dissolution etc, see table 6.2. Multicompartmental tests have been suggested that simulate the sequential dissolution of drugs passing from the stomach through the small intestine /73;74/. A multicompartmental model has been used to demonstrate how a clay mineral impacts dissolution of drugs in the stomach and the upper sections of the small intestine /73/. 6.2.3 Contaminated soil methodsA wide range of methods has been suggested for determining the availability of contaminants, in particular organic contaminants, for soil organisms (mainly bacteria, plants and collembola) and similarly for aquatic ecosystems including both the water and sediment phases: the ecotoxicological bioavailability. The rationale behind these methods is that only the fraction of a contaminant that is present as free compound dissolved in the soil water is available to the soil biota, compare figure 4.2. Conversely, all contaminants that can be dissolved (free and bound) in the aggressive environment of the human stomach and the small intestine will a priori be available for human uptake: be bioaccessible. Therefore, methods developed to measure the ecotoxicological bioavailability are generally not applicable for measuring human bioaccessibility. A survey of methods for in vitro testing of bioaccessibility from soils is presented in table 6.3. It should be emphasised here that comparison of bioaccessibility data from different laboratories might be severely impeded if different methods are used for analysing total concentrations of the soil contaminants. Whereas it is generally accepted that analytical methods for organic contaminants in soils should aim at including the full and total amount of the contaminant, methods are accepted for metals that include only parts of the soil metal contents, e.g.: nitric acid destruction prior to quantification with atomic absorption spectroscopy (AAS) or inductively coupled plasma (ICP) methods /17/. It is therefore recommended always to report the concentration of “total” metals, the concentration of bioaccessible metals (both in mg/kg dw) in addition to the percentage bioaccessibility. In the present report, the impact of using different methods for analysing “total” metal concentrations on percentage bioaccessibilities quoted in this report is not considered.The PBET method was based upon a method developed for estimation of iron bioaccessibility for nutritional research /75/. This method is used in a modified version entitled SBET by the British Geological Survey (BGS) excluding the intestinal digestion step /76/ and by others /43;77-79/. Now, the BGS uses the simple SBET for lead bioaccessibility and PBET for arsenic and other contaminants /80/. Presently, the PBET method is used in a simplified version featuring extraction with glycine buffer at pH = 1,5 and L/S = 100 for 1 hour /7/. This version of the method, called SBRC after the Solubility/Bioavailability Research Consortium, can be extended with a step simulating small intestine digestion: titration to pH = 7,0, addition of bile and pancreatin and digestion for 4 hours. The SBRC does not exclude oxygen during testing. The PBET method has been modified including features from the Digestive tract model and RIVM methods for bioaccessibility testing of polychlorinated dibenzodioxins and –furans /89/. A simpler version including features from the Digestive tract method has been used for bioaccessibility testing of PAH in soil /90/. Furthermore, a modified version of the PBET method has been used for determination of pesticide bioaccessibility in soils /59/. The RIVM method was based upon a method developed by Rotard /91/ for bioaccessibility testing of organic contaminants from mine waste and was used in a slightly modified version by Oomen /2/. The DIN method was also derived from the Rotard method. The TIM model was based upon a dynamic model developed by Minekus simulating the gastrointestinal system for general research purposes /74/. Several of the methods presented in table 6.3 have been used to produce bioaccessibility data as presented in chapter 7. Table 6.3 In development of the published bioaccessibility test methods, a range of important experimental details was identified for specification in a suitable bioaccessibility test method (first record listed is referenced, most findings published by several authors):
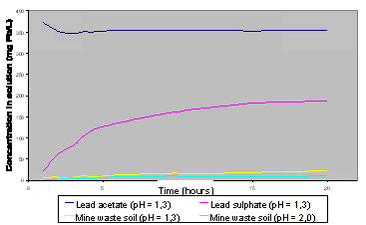
A few additional, important points should be made with respect to test method conditions to support the listing above. In vitro bioaccessibility tests do not include the effects of the microbial communities present in the in vivo gastrointestinal system, and do not include the effects of active transport of contaminants from the digestion solution /17/. It has been demonstrated with sequential extraction that the presence of phosphate changes the speciation of lead towards less extractable species /96/. A study of lead dissolution kinetics under simulated stomach conditions has suggested that a test time of 1 hour is adequate for this compartment /97/. Fast release of lead from contaminated soils (66) and wastes (19) was reported simulating gastric conditions over time /97/, with more than 30% of total lead dissolved within 10 minutes for most samples. The study also demonstrated higher bioaccessibility with lower pH and faster release with higher temperatures. Other studies have suggested a test time of 1,5-2 hours as most appropriate for the stomach simulation /81/. Dissolution of lead minerals in the stomach depend on the acid concentration (figure 6.5) present in the test during dissolution, with low acid concentrations leading to both slower dissolution and lower final dissolved concentration /93/. An average decrease in stomach bioaccessibility of 57% was observed with 7 soils impacted by mine wastes when raising the test pH from 1,3 to 2,5 /82/. The effect of acid concentration is caused by both pH (formation of HSO4-) and chloride concentration (formation of soluble PbCl+) /81/. Arsenic bioaccessibility from 2 soils was lower by 8 - 25% in the PBET test with pH = 2.5 than with pH = 1,3 but the effect was less than for e.g. lead /82/. The pH and dissolution time ranges studied here are comparable to the conditions found in the human stomach (see table 3.1). Recent data suggest that both synthetic stomach fluid and human stomach fluid can significantly reduce toxic and soluble Cr(VI) to less toxic and more insoluble Cr(III) within the time range relevant to stomach transit /98/. Figure 6.5
redrawn and modified after /93/. Figure 6.6
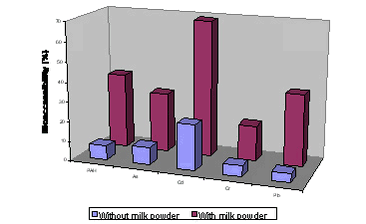
The general effect of adding milk powder to the test system is to enhance dissolution of soil contaminants, as seen for the Digestive tract model in figure 6.6. The effect was also observed for arsenic, cadmium and lead using the DIN test in a method comparison /76/. The increase in PAH bioaccessibility from soil upon addition of milk powder and of mucine to the test system has been reported several times, e.g.: /84/, /99;100/. Also, an increase in metal bioaccessibility has been reported upon factor 6 increase of the concentrations of added mucin, bile and pancreatin in the DIN method /92/, but the effect was not seen consistently for all metals and soils tested, see also figure 6.7 6.2.4 Comparisons of contaminated soil methodsAn interlaboratory comparison of generally applied bioaccessibility test methods demonstrated considerable within laboratory variation (As: 31% mean coefficient of variation, CV, Cd: 34% CV and Pb: 71% CV) and very large between laboratory variation (table 6.4) /76/. Please, note that part of the between laboratory variation may be caused by different pretreatment methods applied prior to bioaccessibility testing. One method is omitted from the evaluation (SHIME), as the method evidently employs a too high pH (4,0) in the gastric digestion step and consequently yields to low results. Differences in pH of the acidic gastric digestion step are given as the most likely explanation of the poor correspondence obtained with the different methods in the interlaboratory comparison, even with the remaining data sets /76/. In particular, the SBET method without a neutral digestion step simulating small intestinal processes gave higher results for the cationic metals (Cd, Pb, compare chapter 4) than did the other methods. The lower results obtained with the combined gastric-intestinal methods are probably caused by precipitation of metals dissolved in the acidic gastric step after neutralisation in the subsequent neutral to alkaline intestinal step Table 6.4
A method comparison has been performed for bioaccessibility of polychlorinated dibenzodioxins and –furans from mine waste with methods similar to the RIVM and the Digestive tract approaches (see table 6.3) /101/. Again, the data demonstrated reasonable precision employing one method but large variations when different methods were used. The main methods differences were use of a mouth and esophagus simulating step, the L/S ratio and the composition of the digestion fluids. Furthermore, an increased bioaccessibility is demonstrated with methods including addition of milk or oil in the digestive steps. Comparison of a method resembling the DIN method, the same method with 6 times higher concentrations of mucine, bile and pancreatine and the Digestive tract model demonstrated that measured bioaccessibility depend entirely upon the specific version of the method used, figure 6.7 /92/. Furthermore, the different versions resulted in higher concentrations for one contaminant and lower concentrations of another. Comparison of methods resembling the RIVM, the GJST and the Digestive tract methods demonstrated that lead mobilisation occurs in the stomach step, but lead is probably demobilised in a subsequent intestinal step due to the higher pH here /102/. The presence of phosphate in the intestinal step may increase the demobilisation as lead phosphate is sparingly water soluble, see chapter 4 and section 6.2.3. Figure 6.7
A comparison of the PBET method with the simpler IVG version demonstrated that bioaccessibility of arsenic from 13 soils impacted by mine wastes was higher with the IVG method. The difference was probably caused by the addition of dough and the higher pepsin concentration used in the IVG method /88/, see table 6.5. Table 6.5  Click on the picture to see the html-version of: ‘‘6.5‘‘ 6.3 Bioaccessibility test performance requirementsAnalytical methods for use in environmental regulation must fulfil minimum requirements with respect to analytical quality or performance. The requirements are generally established in terms of the analytical detection limit (the lowest values that can be detected), accuracy (recovery of true value, “trueness”) and precision (the scatter of replicate data). In Denmark, minimum performance is defined for a number of environmental matrices and analytical parameters /103/, but no requirements have yet been put forward for soil contaminants. The lowest quality accepted is Quality Class 3 that requires:
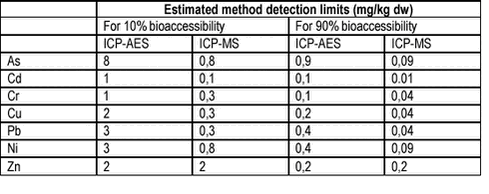
For a test where the result depends upon the precise test conditions, see above in this chapter, no true value can be established. In such cases, the median or mean value obtained by several laboratories with an accepted method is designated the “true value”. Generally, the analytical limit of detection should be lower than 1/10 the maximum contaminant concentration to be controlled. As no maximum limits are currently established for soil bioaccessibility of contaminants, it is suggested to set the limit of detection requirement to 1/10 of the cleanup level. Table 6.6 gives estimated method detection limits for metals with the RIVM test based upon instrument detection limits reported by the Danish commercial laboratory Eurofins A/S for standard analysis (ICP-AES) and for the most sensitive method (ICP-MS). The estimated test method detection limits should be considered lower limits of detection limits, as variability from the test is not included. Also, the test method detection limits will depend upon the test conditions and the analytical set up and performance. For PAH, estimates can not be established similarly, as the volumes of digest available do not correspond to current standard PAH analytical requirements. Table 6.6
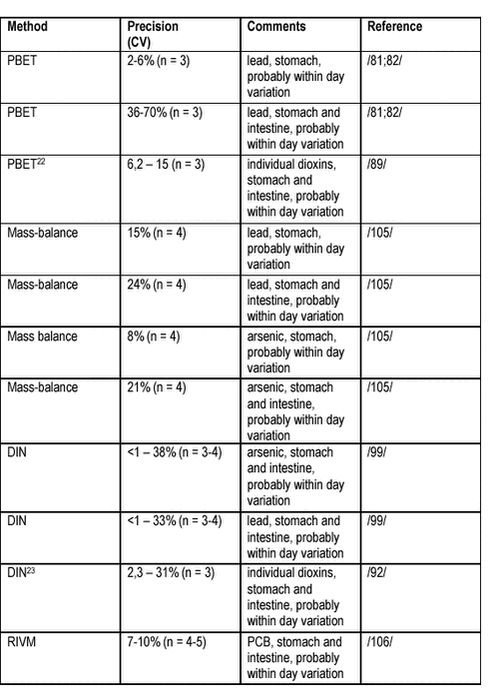
A method detection limit corresponding to 5 mg/kg dw bioaccessible lead has been reported /81/ and a detection limit for 20% bioaccessible As of 5 mg/kg dw has been given /107/. For several studies, the coefficient of variation reported is of the same magnitude as the bioaccessibility measured suggesting that the reported values were in reality below method limits of detection. Within laboratory variation can be estimated for several of the test methods for soil contaminant bioaccessibility described, table 6.7. Overall, fine precision (<7% CV) has been attained for test simulating stomach bioaccessibility of metals from soils, whereas tests simulating stomach and intestine have been used with less precision due to the extra complicating step. Furthermore, precision seems to be worse for lead (precipitating in the intestinal step), and for organic contaminants not as good as for metals such as arsenic. Finally, it should be considered whether the general requirement for a 7% total CV is attainable and necessary analysing a heterogeneous matrix such as soil. No interlaboratory comparisons have been published with one accepted method used and allowing for evaluation of the accuracy of that method and at the participating laboratories. The interlaboratory comparisons published with more than one method employed, see above in this chapter, clearly demonstrate that the requirement for a maximum variation of ±30% from a designated true value is not fulfilled. Table 6.7
6.4 Implications of method studies for bioaccessibility testingA number of bioaccessibility test methods have been developed and details to be specified in a test method has been listed, see section 6.2.3. The conclusions from the method comparisons and studies are that:
It is debatable whether one method suitable for all contaminants (metals and organic compounds) can be found. It has been suggested by the project reference group to use a simple stomach test (SBET, PBET stomach step or SBRC) for Cd, Cu, Pb and Zn, a combined stomach and small intestine test (full PBET, DIN or RIVM) for As and Cr, and a full stomach and small intestine test with bile and food (milk) added for organic contaminants. Test method validation data corresponding to what is generally required for methods to be used for regulatory purposes have not been published. The interlaboratory comparison data required to evaluate a test method have not been published. Realistic test performance requirements should be not higher than Quality Class 3 and method detection limits of 1/10 of MCLs to be enforced, see section 6.3. ____________________________________________________________
|