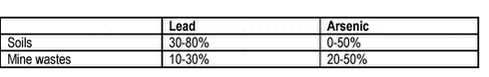
Human Bioaccessibility of Heavy Metals and PAH from Soil8 Bioaccessibility – bioavailability correlations8.1 Heavy metal correlations8.2 PAH correlations 8.3 Overall bioaccessibility – bioavailability correlations Studies of bioavailability (in vivo studies with experimental animals) and bioaccessibility (in vitro dissolution studies simulating the gastrointestinal tract) on the same soils are few. 8.1 Heavy metal correlationsStudies of bioavailability are available for lead and to some degree for arsenic and were reviewed recently /113/. From these studies, a few points should be made regarding bioavailability, although this topic is not part of the current review. The US EPA presupposes a 50% absolute bioavailability of lead from water or food, a 30% absolute bioavailability of lead in soil and thus a relative bioavailability of soil lead of 60% (referred from /113/, see chapter 9). The general picture for the reviewed bioavailability studies is given in table 8.1. It has been suggested that a range of arsenic bioavailability from soils of 8-30% is more appropriate /12/. Assuming 100% bioavailability of soluble As species, this would also be the range for the relative arsenic bioavailability from soils to expect. Table 8.1
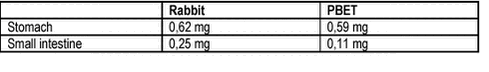
A frequently quoted study of lead uptake in adult humans from mine waste contaminated soil demonstrated 26% absolute bioavailability of ingested lead for fasted individuals and only 1,4% bioavailability in fed individuals /115/. It should be noted that most soils tested are in effect impacted by mine wastes or other metal industry activities. Basically, the 60% relative bioavailablity assumed by the US EPA for lead resembles measured relative bioavailability for most soils, whereas a lower relative bioavailability can be expected for mine wastes. For arsenic, fewer data are available but a relative bioavailability below 50% is to be expected.Recent studies of arsenic, lead and benzo(a)pyrene in soils gave relative bioavailabilities in minipigs of 7-58% (6 soils), 22-72% (6 soils) and 14-39% (4 soils), respectively /99/. Correspondence has been demonstrated between lead bioaccessibility measured with the PBET method (in vitro) and the dissolution process occurring in the rabbit stomach and to some degree also in the small intestine (in vivo), table 8.2. Table 8.2
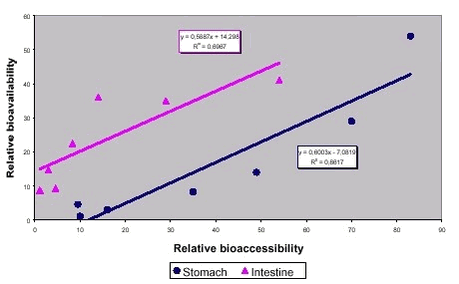
Please, note from figure 8.1 that the relative bioaccessibility from the intestine simulation was lower than the relative bioavailability. This suggests that the precipitation of lead observed after transit from the stomach to the intestinal compartment may not result in reduced uptake. In other words, the test simulating intestinal bioaccessibility produced to low results. Additional data correlating lead bioavailability to bioaccessibility obtained with the stomach part of the PBET test (the SBRC test) demonstrated fine linear correspondence but the same higher bioavailability than bioaccessibility /7/. Figure 8.1
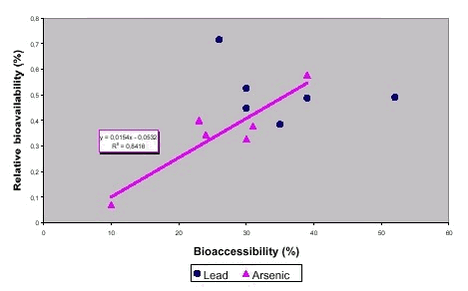
Relative bioavailability in minipigs and bioaccessibility according to the DIN test was measured for arsenic and lead from 6 soils, figure 8.2 /99/. A reasonable correspondence is seen for As, whereas the correspondence is poor for Pb. The poor correspondence for lead is probably caused by a very low bioavailability of lead in the minipig system (3% for soluble lead, 1-2% for soil lead) as compared to the much higher values for arsenic (50% for soluble arsenic, 3-30% for soil arsenic). The quite narrow span of bioavailabilities measured for lead as compared to that for arsenic provide an additional explanation. It should be emphasised here that the slope and the intercept calculated for the linear regression of As in figure 8.2 should be interpreted with caution, because the regression line may be “tilted” by just one value (data point with 10% bioaccessibility). Still, the regression coefficient (R2) of 0,84 demonstrates that a linear relationship between bioaccessibility and bioavailability does explain approximately 84% of the data variability. It is therefore justified to deduce a linear relationship from the data, in spite of the “tilting” effect of one data point. Figure 8.2
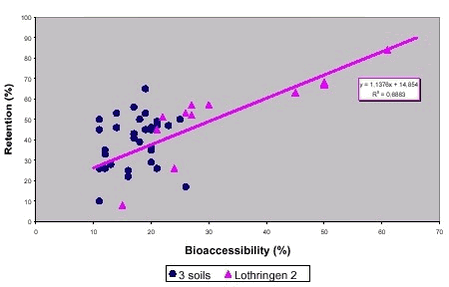
8.2 PAH correlationsBioaccessibilities were obtained for 12 PAH from 4 soils after the DIN method and worst case estimates of bioavailabilities in minipigs were obtained by calculating the fractions of PAH that were not secreted with faeces (the retained fraction, % retention) /99/. The resulting data for retention of PAH overestimate bioavailability as PAH degraded or adsorbed to membranes in the gastrointestinal system are included as bioavailable, see chapter 5. Correlation between measured bioaccessibility and retention is poor (figure 8.3) with all PAH and all soils included. Still for one soil (Lothringen 2), a linear correlation is obviously obtained. This soil is the same where the bioaccessibility pattern suggested reduced PAH bioaccessibility caused by simple absorption into soil organic matter (see chapter 7). Figure 8.3 Figure 8.4
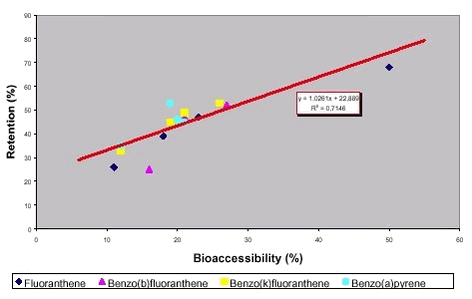
If the correlation between retained fraction and bioaccessibility of this study is considered for one PAH at a time for the 4 soils, a better correlation is obtained. In effect, a reasonable correlation is obtained for 3 of the 7 PAH of this review (fluoranthene, benzo(b)fluoranthene and benzo(k)fluoranthene), an indication of linear correlation for one PAH (benzo(a)pyrene) and no correlation for 2 PAH (dibenz(a,h)anthracene and indeno(1,2,3-cd)pyrene), figure 8.4 (recall comment on linear regression given for figure 8.2). One PAH (benzo(j)fluoranthene ) was not analysed for in this study. That the estimated bioavailabilities are higher than the obtained bioaccessi-bilities suggests that retention indeed overestimates bioavailability. This interpretation is supported by the fact that the amount of PAH eliminated by the primary pathway (metabolites in the urine) is much lower than the amount retained, e.g.: 2,4-13,3% pyrene metabolites excreted with urine, 35-53% pyrene retained in the gastrointestinal system. Alternatively, the bioaccessibility test conditions are insufficient to dissolve all PAH that are made available in the minipig gastrointestinal system. 8.3 Overall bioaccessibility – bioavailability correlationsFor lead, arsenic and PAH, correspondence or linear correlation between bioaccessibility and bioavailability has been reported but the limited data available clearly demonstrate, that the correspondence or correlation is not obtained with all soils and tests. For the remaining metals, no corresponding bioaccessibility and bioavailability data have been found. The correlation between bioaccessibility and bioavailability to be expected depend upon the interactions between dissolution and absorption. Four scenarios can be set, compare chapter 3:
In the equilibrium scenario, bioaccessibility and bioavailability will both be 100%. If the contaminant is present as two species, one dissolving fast and one dissolving very slowly or not at all, the bioaccessibility and bioavailability can be below 100% and still equal. In the scenario with a rate limiting dissolution step, bioaccessibility will be below 100% and will be equal to bioavailability if all conditions of importance for the dissolution processes in the bioaccessibility test correspond exactly to the conditions in the gastrointestinal tract of the experimental animal. A complete correspondence (reagents and their concentrations, reaction times etc.) is probably not attainable and the system may furthermore be highly susceptible to impacts from the soil matrices. Still, if all processes of importance to the dissolution are possible in the test, at linear correlation can be expected between bioaccessibility and bioavailability, see also section 6.2.2 for the approaches taken in drug dissolution testing. In this scenario, measured bioaccessibilities may be higher or lower than bioavailabilities, whereas the relative bioaccessibilites should equal relative bioavailabilities. In the scenario with rate limiting absorption, no correlation between bioaccessibility and bioavailability is to be expected. In the no dissolution/no absorption scenario, bioaccessibility can predict bioavailability of contaminants that are not dissolved, whereas bioaccessibility will not be able to predict bioavailability in cases with no absorption. It should be emphasised here, that the use of experimental animals for both toxicity studies and for bioavailability studies does complicate the interpretations. Interspecies differences in the gastrointestinal system, metabolism, distribution and excretion, as well as in sensitivity will be present. One approach to solving this would be to use tests for bioavailability or bioaccessi-bility in rabbits, if toxicity data were derived from rabbit studies, and to use tests simulating human conditions, if toxicity data were of epidemiological origin. Conversely, we would intuitively prefer to use tests that simulate the human system for correcting estimates of human toxicity. As an operational and practicable approach, this is suggested for the future work. |