Human Bioaccessibility of Heavy Metals and PAH from Soil9 Application of bioavailability in risk assessment, site examples9.1 Rodney Street, Port Colborne, Ontario, Canada9.2 Coal tar distillation plant, US 9.3 Gas manufacturing plant, California, US 9.4 Metal and PAH contaminated sites, US 9.5 A hypothetical application example, As in soil Recently, adjustment of intervention levels has been implemented based upon specific risk assessment including bioavailability studies of metals as soil contaminants. A number of examples are presented below, but the listing should by no means be considered to be complete. Conversely, the use of site specific bioavailability data for polychlorinated dibenzodioxins and –furans in risk assessment of contaminated soils in the US has been opposed by the regulatory community. The reason for this is probably a high level of concern regarding these compounds with both high acute toxicity and severe long term effects (carcinogenesis) /8/. The US EPA integrated exposure uptake biokinetic model, IEUBK, for estimating lead exposure from contaminated soils uses a default oral bioavailability of 30% from soil and 50% from water (i.e.: 60% relative bioavailability of soil lead) /22/. After bioavailability studies, both higher (35-40%) and lower (12-19%) site specific absolute bioavailabilities from soils have been used in risk assessment based upon IEUBK /9/. Overall, 7 of 10 US EPA regions have no guidelines for implementation, 1 region has limited guidelines and 2 officially allows for cleanup level adjustments based upon bioavailability if backed by scientific data /16/. Still, 4-5 EPA regions have accepted use of bioavailability based adjustments, and one has rejected to do so. The general trend in the US is towards accepting bioavailability as one tool in a “weight of evidence” approach, where results obtained with several, each in their own right imperfect, tools are combined to provide sufficient basis for decisions on land use, remediation goals etc /16/. In the UK, bioaccessibility data are used to an increasing extent, in particular for lands with naturally elevated concentrations of metals /80/. For areas with the same, proven history and geology, semi-generic use has been made of bioaccessibility data for adjusting MCLs. In the NL, bioaccessibility data has been used for site specific risk assessment, and considerable efforts are done to expand the data set and to use the data in exposure modelling (for Pb) /116/. 9.1 Rodney Street, Port Colborne, Ontario, CanadaRisk assessment of widespread soil contamination with heavy metals, in particular nickel and lead, caused by a nickel ore processing industry was performed /20/. Basic data are summarised in table 9.1. The combined use of total exposure calculations, health effect compilations and bioaccessibility estimates yielded significant increase in the standard health criterion specifying the level for intervention or further assessment. Table 9.1
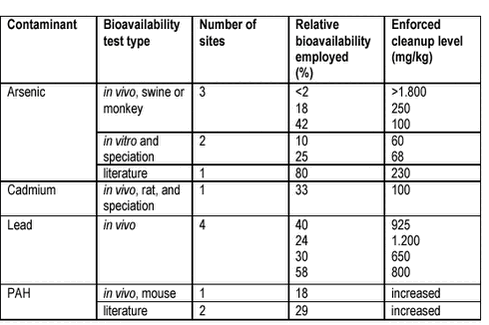
9.2 Coal tar distillation plant, USA combination of literature data and site specific data on bioavailability produced an estimate of oral PAH bioavailability from coal tar contaminated soils of 25-30% /8/. This estimate was used in the final risk assessment of the site. 9.3 Gas manufacturing plant, California, USTo support risk assessment of the site, oral bioavailability (rats), dermal bioavailability, ecotoxicity (earthworms and Microtox) and leaching (SPLP, see chapter 5) was evaluated /8/. The obtained site specific oral bioavailability of PAH of 33% and its use in combination with site specific dermal bioavailability values yielded a factor 5 increase in cleanup levels. It is not clear from the reference, whether the increased cleanup levels were enforced. 9.4 Metal and PAH contaminated sites, USThree reviews summarise the use of bioavailability data in risk assessment of contaminated soils up to Summer 2000 /9;4;16/. Table 9.2 summarises data from these reviews. A number of sites with enforced reductions in cleanup level but with data missing are not included in the table. Table 9.2
In Massachusetts and Michigan, US, oral relative bioavailability factors of 0,5-0,91 has been used for some PAH and petroleum hydrocarbonds, and of 0,5 for As and Cd /16/. Overall, the current experience with site specific use of bioavailability and bioaccessibility test as part of risk assessment is limited but a more widespread use in the future is expected. 9.5 A hypothetical application example, As in soilIn a typical site study, 6-7 samples will be taken for bioaccessibility testing, 1 of which in triplicate, totally 8-9 samples for testing /107/. A control soil sample with known content and bioaccessibility of As and a solution of water soluble As are included in the test series. All samples should be analysed for total arsenic after digestion. In a hypothetical case, total arsenic in the site samples was 50 mg/kg dw ± 3 mg/kg dw (mean ± standard deviation). The test results were 20% ± 5% bioaccessibility in the stomach test and 18% ± 4% in the stomach + intestine test for soil As from the site. The relative standard deviation for the triplicate test was 10%, 13% and 15% for total As, stomach test and stomach + intestine test, respectively. The total As result in the control soil was 53 mg/kg dw (mean of previous data 48 mg As/kg dw ± 4,2 mg/kg dw) with measured bioaccessibilities of 50% and 48% for stomach and stomach + intestine tests, respectively (mean of previous data 45% ± 8,7% and 49% ± 9,5%, respectively). The bioaccessibility measured for the As solution was 103%. As the soil quality criterion (MCL) is derived from toxicity data obtained with readily absorbed As (compare table 2.1), the generic MCL (20 mg/kg dw) can be adjusted using the site specific relative bioaccessibility. The relative bioaccessibility factor, RAcF, for this site can be estimated to 20% based upon similar bioaccessibilities recorded for stomach and for stomach + intestine tests and the ~100% bioaccessibility of the soluble As in the tests done here. The results from analysis and test of the control sample support the validity of the obtained data. The bioaccessible amounts are well above the estimated test method detection limits (compare table 6.6). The variation obtained for the triplicate test of one sample from the site was low and does not indicate an excessive soil inhomogeneity that could disqualify the results. The revised soil quality criterion, MCLrev, for this site would then be calculated as: MCLrev = MCL/RAcF = 20 mg/kg dw/0,2 = 100 mg/kg dw All tested site soil samples were well below the revised soil quality criterion MCLrev. The costs of testing can be estimated for 10 samples (1.500 DKR per sample) to totally 15.000 DKR with an additional 2.000 DKR for test evaluation and commenting. Total costs are estimated to approximately 17.000 DKR for the bioaccessibility study. |