Mapping, emissions and environmental and health assessment of chemical substances in artificial turf
7 Health assessment
7.1 Introduction
This chapter assesses the potential health effects of the identified substances. The assessment is based on worst case scenarios for football players using artificial turf pitches.
For each of the identified and quantified substances, there is information available on the identity and on the chemical and physical properties of the substances. The information concerns state, melting point, boiling point, specific gravity, vapour pressure and solubility.
A review was carried out of the literature via international databases and supplementary searches in scientific literature. As foreign studies have shown that effects caused by inhalation of vapour are insignificant, the focus of the present study was on effects caused by dermal uptake or oral intake. Oral intake may occur by inhalation of, e.g., rubber particles. The most important test results and effects in the literature found are presented. The overall objective has been to find data for NOAEL/LOAEL (No or Low Observed Adverse Effect Levels) on the selected substances or other relevant data available which have been able to contribute further to the assessment.
Based on NOAEL or similar data as well as the amount of substance to which the person is exposed, a margin of safety (MOS) is calculated. Once the margin has been established, it is possible to assess whether the substance has a potentially adverse effect on health when using football pitches installed with the artificial turf mats, infills and pads tested in the study.
7.2 Method
7.2.1 Routes of exposure
The preliminary health screenings identified hazardous substances in both rubber granules and artificial turf material.
The assessment exclusively focuses on the uptake of substances from rubber particles.
The model assumes that the substances can be absorbed (taken up) in the body by oral intake of stirred-up rubber particles or similar inhalation of larger particles which do not reach the lower respiratory passages as well as via the migration of substances from collected rubber particles to the skin via secreted perspiration. It was thus decided not to investigate the inhalation of gaseous substances as the foreign studies have shown that this does not pose a health problem.
7.2.2 Exposure scenarios
There is no information in (TGD 2003) on the amount of rubber particles which are transferred.
Instead, exposure scenarios from foreign studies were used, as described in Section 4.2.4.
It was decided to use worst case scenarios, which means that the maximum exposure levels for rubber particles were used for the calculation of substance exposure.
Skin contact
- Scenario 6: Junior players (16-19 years)
Body weight = 65 kg
Skin surface exposed = 6,600 cm²
Per-exposure duration = 2 hours
No. of exposure episodes per week = 7
Duration in months = 5.5 months
To the above calculation of playing time should be added two two-hour matches every three months. It is assumed that they take place in the same period as the training.
It results in a weekly skin exposure of 49,500 mg of rubber granules or 109 mg of rubber granules per kg of body weight/day (109 mg/kg/day= 49,500 mg per week/7 days/65 kg).
Oral intake
In the Norwegian study (Tore Sander, 2006), the absolute worst case scenario is assumed to be somewhere between 23.7 mg per kg of body weight/day and 93.4 mg of rubber granules per kg of body weight/day for a duration of six months.
The scenarios on which the estimated intake are based take an oral intake of 1.0 g of rubber dust per match as their starting point. It seems unrealistically high because of the unpleasant taste of rubber, but nonetheless serves as a basis for the scenarios.
As worst case scenario, it was decided to assess for 93.4 mg per kg of body weight/day.
Calculation
The exposure scenarios are defined in accordance with EU’s Technical Guidance Document (TGD, 2003).
The skin uptake/oral intake of a substance is calculated as:
I = Q * M * F/BW
Where:
I Intake/uptake per day per kg of body weight
Q Concentration of substance (mg substance/g of sample)
M Amount of substance (g per day) taken in/up
F Fraction of substance absorbed
BW Body weight (kg)
Thus, I = Q * F * 109 mg/kg for skin with the exposure level applied for rubber particles.
Analysis data is only available for leaching of substance into water (µg of leached substance/l of water) over a 24-hour period. These data are used to calculate the substance concentration, Q, as 100 g of rubber granules are used per litre of water. It is assumed that the leached amount of substance per g of granules into water can similarly leach into perspiration and from there through skin.
Q (mg of substance/g) = concentration in leaching tests (mg/l)/100 g.
If there is no data available for dermal uptake, 100% uptake is assumed (F = 1) if the substance log KOW < 4, and 10% uptake (F = 0.1), if log KOW < -1 and log KOW > 4.
If there is no data available for oral intake, 100% uptake is assumed, i.e. F = 1.
Risk assessment
In the assessment of health risks, the calculated exposure, i.e. the uptake, must be compared with NOAEL or similar values. As NOAEL is typically based on animal tests, a margin of safety (MOS) is calculated by dividing NOAEL in mg per kg of body weight with the exposure/uptake level.
If the animal data are based on a chronic long-term high-quality study, the safety factor in the risk assessment is typically 10. The safety factors used to derive a NOAEL for humans are often based on animal tests with, e.g., mice or rats. A factor of 10 is, e.g., used for extrapolation between species (different species) and a factor of 10 to protect sensitive individuals of the species, such as children. If the data are based on LOAEL or a subchronic study, an additional safety factor is added (typically 10). The total safety factor is the total product of the individual safety factors.
In assessing health effects, MOS is not used for sensitising effects, as the effects do not have a lower concentration limit.
7.3 Selected substances
The substances described below have been singled out as being the most significant in terms of health risk when using the products. The substances in question are as follows:
- Benzothiazole
- Dicyclohexylamine
- Cyclohexanamine
- Dibutyl phthalate
The health assessment of the substances is as follows:
7.3.1 Benzothiazole
7.3.1.1 Identity
| Name | Benzothiazole |
| CAS-no. | 95-16-9 |
| EINECS-no. | 202-396-2 |
| Molecular formula | CtH5NS |
Molecular structure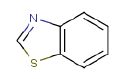 |
|
| Molecular weight (g/mol) | 135.19 |
| Synonyms | |
| Description | Yellow liquid |
| Boiling temperature | 227 ºC (Budavari, 1996) |
| Melting point | 2 ºC (Weast, 1976) |
| Solubility | 3000 mg/l, 25 ºC, water (Iuclid dataset benzothiazole) The substance is slightly soluble in water, alcohol and carbon disulphide (Lide, 1996) |
| Distribution coefficient Log Kow | 2 (Iuclid dataset benzothiazole) |
| Vapour pressure | 0.13 hPa at 20 ºC (Iuclid dataset benzothiazole) |
| Smell | Unpleasant smell (Lewis, 1993) |
7.3.1.2 Amounts found
The substance is found in leaching tests of infill nos. 1, 2, 3 and 14 in concentrations from 245-578 µg/l. There is also a small amount in no. 10 (10 µg/l). The substance is also found by leaching into calcium chloride comparable concentration levels.
7.3.1.3 Function of the substance
The substance originates presumably from degradation products of added mercaptobenzothiazole-based accelerators.
7.3.1.4 Classification and threshold values
The substance is not included in the list of hazardous substances (Danish Ministry of the Environment, 2005), but is included in the guiding list for self-classification (Danish Environmental Protection Agency, 2001) with the specification:
Xn;R22 Harmful if swallowed
R43 May cause sensitisation by inhalation
There is no Danish occupational hygiene threshold value for the substance.
7.3.1.5 Health effects
Data concerning health effects were found in TOXNET and in associated databases. The substance is available as a safety data sheet in IUCLID.
Acute toxicity
Data for acute toxicity:
- LD50 rat, oral 177-479 mg/kg (IUCLID data set benzothiazole, 2003)
- LD50 rat, dermal 500 mg/kg (IUCLID data set benzothiazole, 2003)
- LD50 rabbit, dermal 126-400 mg/kg (IUCLID data set benzothiazole, 2003)
Subchronic toxicity
Some benzothiazoles of the type mercaptobenzothiazoles are sensitising, but no data was found from animal tests for benzothiazole. Data were, however, found which show an allergenic effect on a 10-year-old girl (Contact dermatitis 2007, v57, p 56).
The substance has not exhibited genetic toxicity in Ames test or in other bacterial mutation tests (IUCLID data set benzothiazole, 2003).
Chronic toxicity
No relevant data were found (IUCLID data set benzothiazole, 2003).
7.3.1.6 Exposure scenarios
The maximum benzothiazole content in liquid from leaching is 578 µg of benzothiazole per litre of liquid containing 100 g of rubber granules. Of this is calculated an expected maximum leached amount of substance of 5.78 µg of substance/g of rubber particles per day.
With a log KOW = 2, the skin absorption is assumed to be 100%.
For skin uptake, a maximum exposure to rubber granules of 109 mg/kg/day is assumed, which gives:
Uptake, skin = 0.109 g/kg * 1 * 5.78 µg/g = 0.63 µg/kg of body weight/day.
Oral intake is at the same level in worst case scenario:
Intake, oral = 0.093 g /kg * 1 * 5.78 µg/g = 0.54 µg/kg of body weight/day.
7.3.1.7 Assessment
Health data for assessment
The substance may be allergenic to some individuals, but there are no data for the frequency.
There are no data for long-term effects in animals, for which reason the lowest acute toxicity is used for the assessment (LD50 rabbit, dermal 126 mg/kg/day).
Health risk
Based on LD50 rabbit, dermal 126 mg/kg/day a safety margin of MOS = 126/0.00063 = 200,000 is obtained. MOS for oral intake is at the same level. As the health effect is acute, an uncertainty factor of at least 10,000 should be used in risk assessment. MOS is approx. 20 times above the uncertainty factor, and it is thus assessed that there is no risk of toxic effects. The data basis is, however, very limited.
It is assessed that there may be an allergy risk for sensitive individuals.
7.3.2 Cyclohexanamine
7.3.2.1 Identity
| Name | Cyclohexanamine |
| CAS-no. | 108-91-8 |
| EINECS-no. | 203-629-2 |
| Molecular formula | C6H13N |
Molecular structure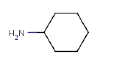 |
|
| Molecular weight (g/mol) | 99.18 |
| Synonyms | Cyclohexylamine |
| Description | Colourless or yellow liquid |
| Boiling temperature | 134.5 ºC (Budovari,1996) |
| Melting point | -17.7 ºC (Budovari,1996) |
| Solubility | The substance is mixable with water 20 ºC (Iuclid dataset cyclohexylamine). Is highly soluble in ethanol and mixable in ether, acetone (Lide, 1996) |
| Distribution coefficient Log Kow | 1.49 (Hansch, 1995) |
| Vapour pressure | 14 hPa at 20 ºC (Iuclid dataset cyclohexylamine) |
| Smell | Strong fishy amine smell (Budovari, 1996) |
7.3.2.2 Amounts found
The substance is found in leaching tests of infill no. 14 in a concentration of 1610 µg/l. In calcium chloride, the concentration is lower with 533 µg/l.
7.3.2.3 Function of the substance
The substance is presumed to be a dissociation product from an added accelerator.
7.3.2.4 Classification and threshold values
The substance is included in the list of hazardous substances (Danish Ministry of the Environment, 2005) and is classified as:
R10 Flammable
Xn;R21/22 Harmful in contact with skin and if swallowed
C;R34 Causes burns
The threshold value for working environment is 10 ppm or 40 mg/m³ (Danish Working Environment Authority (WEA) guide, 2007).
7.3.2.5 Health effects
Data concerning health effects were found in TOXNET and in associated databases. The substance is available as a safety data sheet in IUCLID.
Acute toxicity
Data for acute toxicity:
- LD50 rat, oral 136-496 mg/kg (IUCLID data set cyclohexylamine)
- LD50 mouse, oral 224 mg/kg (IUCLID data set cyclohexylamine)
- LD50 rabbit, dermal 208-372 mg/kg (IUCLID data set cyclohexylamine)
It is a weak methemoglobin-forming substance (American Conference, 1991).
Subchronic toxicity
The substance is specified as a moderately sensitising substance (American Conference, 1991). It is also specified (Lewis, 1996) that the substance may cause dermatitis.
The substance has a neurotoxic effect. Tests with oral doses to mice thus showed increased movement activity after one hour at 37, 74 and 148 mg/kg with an increase of 41% at 148 mg/kg (IR PRODS & CHEM CO 7/2/87).
Chronic toxicity
A 90-day feeding study in 2 * 25 rats in doses (start/end): 75/30, 227/100 and 525/296 mg/kg showed weight loss and reduced food intake in the two highest dose groups as well as a 80% reduction in sperm production in the highest dose group (reprotoxic effect) (IUCLID data set cyclohexylamine).
Another 90-day feeding study in 100 rats in each group with 50, 100, 200 and 300 mg/kg showed weight loss and reduced sperm production in the two highest dose groups.
The NOAEL determined for this study was 100 mg/kg (IUCLID data set cyclohexylamine).
A five-generation study in rats shows an effect on the reproductive ability from 100 mg/kg, but no mutagenic or teratogenic effects up to the highest dose of 150 mg/kg/day for the F0 generation. No effects were observed at 50 mg/kg/day (IUCLID data set cyclohexylamine).
A study with six dogs per group was carried out over 9.5 years. The dose during the first four years was 0.15, 1.5 and 15 mg/kg/day, respectively. In the remaining period, the dose was 50, 100 and 150 mg/kg/day, respectively. No health effects were found during the first four years, but a weight loss was observed when changing dose. The weight was, however, slowly regained, and no other effects were observed during the study period (IUCLID data set cyclohexylamine).
Two-year feeding studies in 2 * 25 rats with oral doses, 0.15, 1.5 and 15 mg/kg, respectively, as well as 30-month feeding studies in 2 * 52 rats with 200 mg/kg show no significant carcinogenic effects, the level of effects in test animals being the same as in the control group (IUCLID data set cyclohexylamine).
No teratogenic effects were observed in feeding studies in rats on days 7-13 of the pregnancy at doses up to 36 mg/kg/day and no teratogenic effect in studies in pregnant rhesus monkeys on days 20-45 of the pregnancy at doses up to 75 mg/kg (IUCLID data set cyclohexylamine).
A reference dose exists for the substance in IRIS (TOXNET database) based on a two-year study in 2 * 48 rats as well as a six-generation feeding study in rats where NOAEL determined for testicular damage in rats was 18 mg/kg/day with a LOAEL of 60 mg/kg/day.
With an uncertainty factor of 100, a reference dose of RfD = 0.2 mg/kg/day is obtained.
7.3.2.6 Exposure scenarios
The maximum content in liquid from leaching is 1,610 µg cyclohexanamine per litre of liquid with 100 g of granules. Of this is calculated an expected maximum leached amount of substance of 16.1 µg of substance/g of rubber particles per day.
With a log KOW = 1.49, the skin absorption is assumed to be 100%.
For skin uptake, a maximum exposure to rubber granules of 109 mg/kg/day is assumed, which gives:
Uptake, skin = 0.109 g /kg * 1 * 16.10 µg/g = 1.75 µg/kg of body weight/day.
At an exposure of a maximum of 93 mg/kg/day, the uptake by oral intake will be at a level comparable to that of skin uptake.
7.3.2.7 Assessment
Health data for assessment
Data show that the substance is not carcinogenic, harmful to reproduction or mutagenic.
The substance is moderately sensitising.
NOAEL for rats is 18 mg/kg/day, and Rfd = 0.2 mg/kg/day.
Health risk
Based on the NOAEL for rats of 18 mg/kg/day, a safety margin of MOS = 18/0.00175 = 10,200 for skin uptake is obtained, which is approx. 100 times above the uncertainty factor of 100 specified in the calculation of reference dose. MOS for oral intake is at the same level.
Exposure to the substance is not assessed to be associated with health effects with the exception of particularly sensitive individuals who may develop allergic reactions.
7.3.3 Dicyclohexylamine
7.3.3.1 Identity
| Name | Dicyclohexylamine |
| CAS-no. | 101-83-7 |
| EINECS-no. | 202-980-7 |
| Molecular formula | C12H23N |
Molecular structure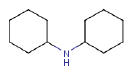 |
|
| Molecular weight (g/mol) | 181.31 |
| Synonyms | Cyclohexanamine, N-cyclohexyl- |
| Description | Colourless liquid |
| Boiling temperature | 255.8 ºC (Budovar, 1996i) |
| Melting point | -0.1 ºC (Budovari, 1996) |
| Solubility | 800 mg/l, 25 ºC, water (Iuclid dataset dicyclohexylamine). The substance is soluble in ethanol, ether, benzene (Lide, 1996) |
| Distribution coefficient Log Kow | 3.5 (Iuclid dataset dicyclohexylamine) |
| Vapour pressure | 1 hPa ved 65 ºC (Iuclid dataset dicyclohexylamine) |
| Smell | Aminelike smell (Ashford, 1994) |
7.3.3.2 Amounts found
The substance is found in leaching tests of infill no. 1, 2, 3 and 14 in concentrations from 99 to 1,167 µg/l. The substance appears to be converted in the alkaline calcium chloride, e.g. by oxidation, which means that the concentration drops to 1/10-1/100 as compared to leaching with neutral water.
7.3.3.3 Function of the substance
The substance is presumed to be a dissociation product from an added accelerator.
7.3.3.4 Classification and threshold values
The substance is included in the list of hazardous substances (Danish Ministry of the Environment, 2005) and is classified as:
Xn, R22 Harmful if swallowed
C;R34 Causes burns
N; R50/53 Environmentally hazardous substance; Very toxic to aquatic organisms/
May cause long-term adverse effects in the aquatic environment
There is no occupational hygiene threshold value for the substance.
7.3.3.5 Health effects
Data concerning health effects were found in TOXNET and in associated databases. The substance is available as a safety data sheet in IUCLID.
Acute toxicity
Data for acute toxicity:
- LD50 rat, oral 200-373 mg/kg (IUCLID data set dicyclohexylamine)
- LD50 mouse, oral 500 mg/kg (IUCLID data set dicyclohexylamine)
- LD50 rabbit, dermal 200-316 mg/kg (MONSANTO CO, 03/18/77)
Tests in rabbits show that the substance causes burns at 45 mg/animal for 8 hours and severely irritating at 0.75 mg/animal for 24 hours (IUCLID data set dicyclohexylamine).
(E. Goettinger, 1971) states that 40% of dicyclohexylamine is converted into cyclohexanamine.
Subchronic toxicity
No test data showing a sensitising effect on animals have been found. In (Budovari, 1996), the substance is, however, listed as a potentially sensitising substance.
Chronic toxicity
No long-term test data have been found, but data for acute toxicity show that the acute toxicity is comparable with that of cyclohexylamine within a factor of 2-3.
As 40% of dicyclohexylamine is converted into cyclohexanamine, it is presumed that NOAEL for dicyclohexylamine is at the same level as for cyclohexanamine, corresponding to approx. 20 mg/kg/day. In the assessment, data for acute toxicity are, however, used.
7.3.3.6 Exposure scenarios
The maximum content in liquid from leaching is 1,167 µg cyclohexanamine per litre of liquid with 100 g of granules. Of this is calculated an expected maximum leached amount of substance of 11.67 µg of substance/g of rubber particles per day.
With a log KOW = 3.5, the skin absorption is assumed to be 100%.
For skin uptake, a maximum exposure to rubber granules of 109 mg/kg/day is assumed, which gives:
Uptake, skin = 0.109 g /kg * 1 * 11.67 µg/g = 1.27 µg/kg of body weight/day.
At an exposure of a maximum of 93 mg/kg/day, the uptake by oral intake will be at a level comparable to that of skin uptake.
7.3.3.7 Assessment
Health data for assessment
The data for the substance only comprises acute toxicity with the lowest acute toxicity LD50 rat, oral = 200 mg/kg/day.
The substance may be sensitising.
Health risk
A calculation based on the lowest acute toxicity LD50 rat, oral = 200 mg/kg/day gives MOS = 200/0.00127 = 157,000 for skin uptake, which is 16 times above an uncertainty factor of 10,000, which should be used in the assessment of acute toxicity. MOS for oral intake is at the same level.
Exposure to the substance is not assessed to be associated with health effects with the exception of particularly sensitive individuals who may develop allergic reactions.
7.3.4 Dibutyl phthalate
The substance has been described in (Mapping no. 43, 2003). The following data have been updated.
7.3.4.1 Identity
| Navn | Dibutylphtalate |
| CAS-no. | 84-74-2 |
| EINECS-no. | 201-557-4 |
| Molecular formula | C16H22O4 |
Molecular structure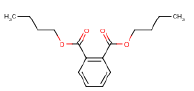 |
|
| Molecular weight (g/mol) | 278.34 |
| Synonyms | |
| Description | Oily liquid (Rar dibutylphtalate) |
| Boiling temperature | 340 ºC (Rar dibutylphtalate) |
| Melting point | -69 ºC (Rar dibutylphtalate) |
| Solubility | 10 mg/l, 20 ºC, water (Rar dibutylphtalate) |
| Distribution coefficient Log Kow | 4.57 (Rar dibutylphtalate) |
| Vapour pressure | 9.7 hPa ved 55 ºC (Rar dibutylphtalate) |
| Smell | - |
7.3.4.2 Amounts found
The substance is found in leaching tests of all studied infill nos. 1, 2, 3, 4, 10 and 11 in concentrations from 61-178 µg/l. The substance is degraded (hydrolysis of ester binding) to < 5% of the concentration in neutral water in all the alkaline calcium chloride-based contact liquids (nos. 1, 3, 11 and 14).
7.3.4.3 Function of the substance
The substance is a softener which may be a contaminant, or which may originate from an adhesive.
7.3.4.4 Classification and threshold values
The substance is included in the guiding list for self-classification (Danish Ministry of the Environment, 2005) and is classified as:
N;R51/R53 Environmentally hazardous substance; Toxic to aquatic organisms/May cause long-term adverse effects in the aquatic environment.
In (Rar dibutyl phthalate), the substance is classified as:
T;R61,R62 Toxic; May cause harm to the unborn child/Possible risk of impaired fertility
N; R50 Environmentally hazardous substance; Very toxic to aquatic organisms
The working environment threshold value for the substance is 3 mg/m³ (Danish Working Environment Authority (WEA) guide, 2007).
7.3.4.5 Health effects
The data are based on the risk assessment for the substance (Rar dibutyl phthalate).
Acute toxicity
The acute toxicity is very low:
- LD50 rat, oral 6,300-8,000 mg/kg (Rar dibutyl phthalate)
- LD50 mouse, oral 4,840 mg/kg (Rar dibutyl phthalate)
- LD50 rabbit, dermal 20,000 mg/kg (Rar dibutyl phthalate)
Subchronic toxicity
The substance has not been found to be sensitising in animal tests (Rar dibutyl phthalate).
In a three-month feeding study in rats, a NOAEL of 152 mg/kg/day for hepatic effects (peroxisomal-based hepatic changes) was obtained.
Chronic toxicity
According to (Rar dibutyl phthalate), there are no data to decide whether the substance has carcinogenic effects.
As for reprotoxic effects, a one-generation study in rats gave a NOAEL of 50 mg/kg/day for teratogenic effects, whereas a two-generation study gave a LOAEL of 52 mg/kg/day for teratogenic effects for male rats and 80 mg/kg/day for female rats, with a maternal toxicity of 375 mg/kg/day.
In (Rar dibutyl phthalate), the LOAEL determined for teratogenic effects was thus 52 mg/kg/day based on the two-generation study, whereas a NOAEL of 152 mg/kg/day is used for non-pregnant adult rats.
7.3.4.6 Exposure scenarios
The maximum content in liquid from leaching is 178 µg dibutyl phthalate per litre of liquid with 100 g of granules. Of this is calculated an expected maximum leached amount of substance of 1.78 µg of substance/g of rubber particles per day.
With a log KOW = 4.57, the skin absorption is assumed to be 10%.
For skin uptake, a maximum exposure to rubber granules of 109 mg/kg/day is assumed, which gives:
Uptake, skin = 0.109 g/kg * 0.1 * 1.78 µg/g = 0.019 µg/kg of body weight/day.
For oral intake, 100% uptake is assumed.
Intake, oral = 0.093 g/kg * 1 * 1.78 µg/g = 0.16 µg/kg of body weight/day.
7.3.4.7 Assessment
Health data for assessment
The substance causes hepatic damage in rats at a NOAEL of 152 mg/kg/day and has teratogenic effects at a LOAEL of 52 mg/kg/day.
Health risk
A LOAEL for rats of 52 mg/kg/day gives a safety margin of MOS = 52/0.00016 = 314,000 for oral intake. The MOS for skin uptake is approx. ten times higher. With an uncertainty factor of 1,000 based on the LOAEL, it is thus deemed that there are no health effects associated with exposure to the substance.
7.3.5 Conclusion
The overall assessment is that there are no health effects associated with exposure to the four substances tested, with the exception of a potential risk for developing allergy in particularly sensitive individuals (benzothiazole and the two amines).
Version 1.0 October 2008, © Danish Environmental Protection Agency