Dermal absorption of pesticides - evaluation of variability and prevention
7 Effect of solubility and molecular size on skin penetration
- 7.1 Determining percutaneous penetration rate
- 7.2 Relation between Kρ and logPow
- 7.3 Association between lag-time and solubility
- 7.4 The relation between the lag-time and the molecular weight
- 7.5 Skin deposition and substance solubility
- 7.6 Molecular size in relation to substance solubility and deposition
- 7.7 Exposure in relation to occupational risk assessment
The dermal absorption is influenced by the solubility of the pesticide - a high lipophilicity as well as a high hydrophilicity limit the skin penetration (McDougal & Boeniger, 2002). This is an important consideration when designing drugs for topical use, cosmetics and also when designing pesticides. Pesticides need an effective penetration when it comes to the outer membrane of the target organism (plants or pests). Knowledge about the fastest or optimal penetration is also significant when estimating human risk and the ability to secure prevention.
Substances that are intended to cross the skin must have a molecular weight below 1000 daltons. The size is probably not the limiting factor but with increasing size the chemical structure becomes more complex, the partitioning behaviour changes and penetration is therefore reduced. The skin is a multi-complex membrane and changes from an avascular and lipophilic structure (stratum corneum) to a more aqueous structure (the viable epidermis and dermis). Uncomplicated penetration of a substance requires both solubility in the lipophilic environment and the more aqueous environment (Guy et al., 1987).
7.1 Determining percutaneous penetration rate
The objective of this part of the report is, through results on percutaneous penetration of different test substances, to determine an interval in penetration ability in relation to the substance solubility and hopefully find an interval where the most effective percutaneous penetration takes place. The selected substances represent a broad interval of solubilities as well as a relevant interval in molecule weights.
Nielsen et al. have tested glyphosate, benzoic acid, malathion, caffeine, pirimicarb, methiocarb, paclobutrazol, dimethoat and prochloraz and characterized these substances by their solubility, logPow, molecular weight, Kρ and lag-time as shown in Table 1 (Nielsen JB et al., 2006;Nielsen et al., 2004). The potential to create a reservoir is in the table indicated by the amount of substance in the epidermis and dermis at the end of experiments. The total penetration is together with the unabsorbed amount of test substance in the donor chamber, and the amount remaining in the skin.
Experimental data on percutaneuos penetration rate (flux) are obtained by measurements of concentrations in the receptor chamber over time. This measurement is used to generate the apparent Kρ-(permeability coefficient) by dividing the steady-state flux obtained in experiments with infinite dosing with the concentration in the donor chamber. For occupational risk assessment following dermal exposure, the flux or relative absorption (percentage absorption per day of an occupationally relevant dose) is often used to estimate the risk. The penetration rate is, however, not in its own sufficient to evaluate the toxicity profile of a pesticide after dermal exposure. Thus, two pesticides with significantly different maximal flux, and therefore also different Kρ’s, may cause identical pesticide doses given their lag-times also differ. Except for in vitro studies with continuous sampling (flow-through cells), lag-times are often difficult to measure and will most often be estimated based on a back extrapolation from the linear part of the penetration curve (see Figure 4 in section 3.2.1).
Table 1. Solubility and penetration characteristics of 9 test substances. Experiments were based on static diffusion cells mounted with human skin, carried out under comparable conditions, and terminated at 48 hours.
| Relative deposition | ||||||||||
| Solubility | logPow | MW | Kρ | lag-time | receptor | epidermis | dermis | donor | recovery | |
| (g/L) | (g) | (um/h) | (h) | (%) | (%) | (%) | (%) | (%) | ||
| Glyphosat | 12 | -1.7 | 170 | 0.06 | 7.9 | 0.4 | 0.5 | 0.2 | 91.0 | 92.0 |
| Caffein | 21.7 | 0.16 | 194 | 3.8 | 6.9 | 18.7 | 3.4 | 3.6 | 72.8 | 99.6 |
| Dimethoate | 23.8 | 0.7 | 229 | 4.9 | 22.3 | 5.1 | 0.4 | 1.5 | 104.5 | 109.4 |
| Pirimicarb | 3 | 1.7 | 238 | 27.7 | 15.2 | 34.1 | 1.5 | 9.7 | 52.5 | 96.3 |
| Benzoic acid | 3 | 1.83 | 122 | 50.6 | 1.5 | 93.4 | 0.4 | 1.1 | 4.2 | 99.0 |
| Malathion | 0.15 | 2.75 | 330 | 1.9 | 2.9 | 11.6 | 2.7 | 5.7 | 71.4 | 91.4 |
| Paclobutrazol | 0.026 | 3.2 | 294 | 29.2 | 18.4 | 23.5 | 3.7 | 17.0 | 41.1 | 84.1 |
| Methiocarb | 0.027 | 3.34 | 225 | 38.6 | 11.7 | 50.9 | 2.4 | 23.5 | 17.1 | 94.4 |
| Prochloraz | 0.034 | 4.4 | 377 | 17.8 | 21.1 | 13.7 | 4.2 | 27.5 | 58.3 | 101.7 |
The diffusion process across the skin is believed to be passive and the experimental conditions should reflect infinite dosing. Thus, the maximal flux characterizes a steady-state situation and will therefore represent the slowest of the two rate constants for penetration (in or out of the skin compartment). These rate constants depend on molecular size and lipophilicity of the substance as well as the lipophilicity difference between the matrix that the substance is leaving to the matrix that the substance is entering. If the test substance has a preference for the skin compared to the receptor, a higher concentration gradient will be needed to get substantial transfer into the receptor compartment, and the potential for a reservoir exists. The lag-time is therefore also depending on the existence of a reservoir in the skin barrier, whereas the flux in infinite dose studies at steady state will be independent of the existence of a reservoir effect. Moreover, the thickness of the skin membrane affects the distance that a substance will have to pass through and thus the lag-time.
7.2 Relation between Kρ and logPow
The most efficient absorption is a combination of high penetration rate, illustrated as a high Kρ, and a short lag-time. The amount of substance found in the skin - the epidermis and dermis, is likewise of importance as this reflects the amount of substance which is available for subsequent penetration.
The results from the studies by Nielsen et al. (2004, 2006) show that the lowest penetration coefficient (Kρ) reflects the most hydrophilic substance (glyphosat) and the hydrophilicity of the substance decreases as the Kρ gets higher (Table 1). The maximum Kρ is seen for benzoic acid with a Kρ almost a 1000-fold higher than Kρ of glyphosate. The difference in hydrophilicity expressed as the octanol/water ratio (Pow) is more than 3000 which corresponds to a difference in logPow of just around 3. As for the 3 most lipophilic test substances (methiocarb, paclobutrazol, prochloraz), the Kρ is lower than for benzoic acid. The decrease of logPow in relation to benzoic acid is, however, not as significant for the most lipophilic substances as for the most hydrophilic substances. There is a clear relation between logKρ and logPow. (Figure 11).
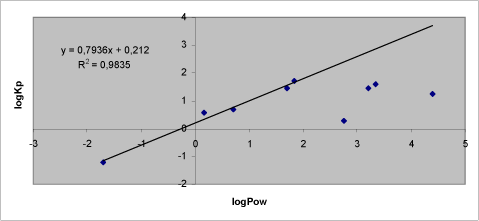
Figure 11. Correlation between logPow and logKρ for nine test substances. Trend line is based on chemicals with logPow below 2.
This is in good agreement with previous studies describing the optimal penetration through the skin of substances with a logPow around 2 (Morgan et al., 2003;Cross et al., 2003) and a recent study demonstrating a fast penetration into the lipophilic stratum corneum but then a more resistant penetration into the underlying hydrophilic part of the skin by didecyldimethylammonium chloride logPow 4.7 (Buist et al., 2007).
In their previous studies Nielsen et al. did not demonstrate a clear relation between molecular weight and penetration rate expressed as Kρ. This is rather surprising given previous literature studies based on larger databases of studies involving dermal penetration, which show a direct relation between molecular weight and penetration rate (Magnusson et al., 2004;Tsai et al., 2003). However, when taking a closer look at these studies of 87 test substances they show large variations and quite a few of the substances are removed from the regression analysis because of deviation compared to the other substances in the studies. In comparison to the studies of Nielsen et al. in 2004 and 2006 who found one substance (malathion) with an unexpected penetration profile, they may well have found other deviating substances had they included more substances in their study. If they had done so they might have found an association between their results and the Australian literature study by Magnusson et al. (Magnusson et al., 2004).
Based on the solubility and molecular weight of malathion, it was expected that the penetration characteristics would place this substance between methiocarb and benzoic acid. As seen from Figure 11 and Table 1, this is not the case. A thorough examination of the experimental set-up, analytical method etc. has revealed no obvious explanation. The most likely explanation is that structural circumstances cause the deviating penetration characteristics of malathion compared to other substances.
7.3 Association between lag-time and solubility
The amount of a substance that in a given time is absorbed through the skin is also affected by the lag-time of the substance. The lag-time will depend not only on the solubility but also on the size of the substance. There is a relationship between lag-time and solubility of a substance. A substance with a fast penetration rate is also expected to pass through the skin after a shorter lag-time than a substance with a slow penetration rate (Figure 12 and 13) (Nielsen JB et al., 2006).
Figure 13 indicates that a short lag-time is to be expected of substances with water solubilities between 3g/L and around 20g/L.
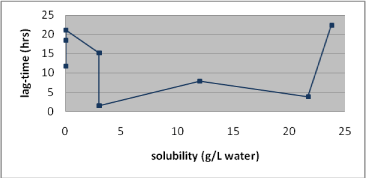
Figure 12: Association between the solubility in water and the lag-time of nine test substances.
7.4 The relation between the lag-time and the molecular weight
There is a clear relationship between the molecular weight and observed lag-time of most of the test substances in the study by Nielsen et al. When the molecular weight increases the lag-time does the same (Figure 13) (Nielsen JB et al., 2006).
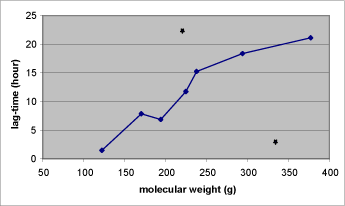
Figure 13: Relation between the molecular weight and the lag-time of the nine test substances described in Table 1. The lag-time of malathion (below) and dimethoat (above) is marked with stars.
The smallest test substance in the study by Nielsen et al. had a molecular weight of 122 g and showed a lag-time of 1½ hours. In comparison with substances with weights above 350 g a clear increase in lag-time was observed. The lag-time increases to more than 20 hours for some of the substances with high molecular weights. The significant difference in lag-time affects the amount of a substance penetrating in a limited period of time. This is highly relevant in occupational risk assessments since skin absorption on a normal working day of 6-8 hours will depend on the substance lag-time.
When evaluating the molecular weight in relation to the lag-time it is also here found that malathion acts unexpectedly by having a lag-time of only 3 hours when having a weight of 330g – a lag-time which would be expected of a substance with half the weight (Nielsen JB et al., 2006).
7.5 Skin deposition and substance solubility
The deposition in the epidermis is not surprisingly relatively limited since it is only the stratum corneum that is involved. The differences in epidermis deposition indicate a relatively high deposition of the most lipophilic substances even though caffeine differs by a relatively high deposit of around 3%.
In the dermis there is a greater relative deposit of the lipophilic substances which was also to be expected as the dermis is a predominant lipophilic matrix. Analogous results are shown in a study including 5 different alcohols with different solubility characteristics (Cross et al., 2003).
The amount of test substance deposit in the donor chamber after end exposure time is as expected greatest among the more hydrophilic substances as they show poor affection of the more lipophilic compartments in the skin (Figure 14).
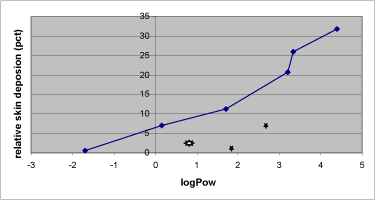
Figure 14: Association between the lipophilicity expressed as logPow and the relative skin deposition of the nine test substances in Table 1. Excluded from the line drawing is from left to right: dimethoate, benzoic acid, malathion.
Even though dimethoat has the same molecular weight as methiocarb, the solubility characteristics of dimethoat were a 1000-fold higher water solubility. Not only does the high water solubility prevent a dermal penetration, but it also prevents a significant amount of pesticide to even enter the more lipophilic epidermis. Despite of the high water solubility in pimicarb, this substance had difficulty depositing in the skin compartment, even though it did penetrate the skin with great flux when compared with methiocarb having the same weight as pirimicarb. Thus, 13% of the pirimicarb dose penetrated the dermal barrier in the experiment with 24 h pesticide exposure when compared with only 8.7% of the methiocarb dose. The high flux and low skin deposition of pirimicarb, when compared with methiocarb, signify the lack of a longer lasting skin reservoir of pirimicarb, and a limited accumulation during the penetration process. This conclusion is based on the observation that penetration into the receptor chamber continues for at least 6 h after pirimicarb is removed from the donor chamber, the low flux during the last part of the experimental period, and the very low skin deposition at 48 h (Nielsen et al., 2004). These observations on skin deposition are also in agreement with the lower lipophilicity of pirimicarb when compared with methiocarb and the relatively low lag-time compared with the other pesticides.
7.6 Molecular size in relation to substance solubility and deposition
The influence of molecular size has been compared using three pesticides (methiocarb, paclobutrazol and prochloraz) with similar solubilities, but different molecular weights (Nielsen & Nielsen, 2000;Nielsen et al., 2004). The flux and consequently the calculated Kρ values for methiocarb and paclobutrazol were almost identical irrespective of whether the exposure period was 6 h or 24 h, whereas flux as well as Kρ for prochloraz (the pesticide with the highest molecular weight) was lower.
The amount of pesticide penetration during the 48 h experimental period decreased with increasing molecular weight irrespective of exposure periods. Likewise, a significantly (P< 0.0 l) higher fraction of the administered dose was removed from the donor chamber in experiments with methiocarb (Mw = 225 g/mol) when compared with paclobutrazol (Mw = 293 g/mol). The significantly higher removal of pesticide from the donor chamber was also reflected through a higher skin deposition in experiments with methiocarb when compared with experiments with paclobutrazol. Prochloraz, on the other hand, had the lowest total penetration but a distribution between donor and skin compartments that was more like methiocarb than paclobutrazol, although prochloraz had the largest molecular weight. Despite equal solubilities in water, the logPow value of prochloraz was higher than logPow for methiocarb and paclobutrazol, which could indicate a higher reservoir preference for prochloraz when compared with the more hydrophilic donor and receptor solutions. The apparent reservoir preference of prochloraz is most significantly reflected through the distribution in the experiment with 24 h exposure, where more than 90% of the administered dose was estimated to remain in the skin compartment when the experiment terminated after 48 h (Nielsen et al., 2004).
Based on the data from Nielsen et al. 2004, it can be concluded that solubility characteristics significantly affect penetration rates as well as skin deposition, and that a too high as well as a too low lipophilicity may limit the rate and degree of skin penetration. These observations pertain to studies on full-thickness skin, in which a significantly larger potential for skin deposition (reservoir) exists. Several mathematical models have been developed to describe the relationship between dermal penetration and the underlying physical parameters such as logPow and molecular weight (Guy & Potts, 1993;McKone & Howd, 1992;Wilschut et al., 1995). Equal for these theoretical models is their unidirectional dependence on molecu1ar weight and logPow as well as their lack of dependence upon experimental conditions related to skin thickness and choice of receptor fluids. The observation that a too high as well as a too low lipophilicity limits dermal penetration is thus at variance with those models. A possib1e explanation might be that these models do not sufficiently consider the penetration as a process involving penetration from a hydrophilic donor to a more lipophilic membrane as well as from the lipophilic membrane and into a more hydrophilic receptor. In the first process, penetration of hydrophilic compounds (like dimethoat) will be limited, and in the second process, more lipophilic compounds (like prochloraz) will prefer to remain in the lipophilic skin compartment.
7.7 Exposure in relation to occupational risk assessment
To mimic an occupational exposure situation Nielsen et al. have studied skin penetration after 6 h exposure time (Nielsen et al., 2004). The use of infinite dosing is at variance with most occupational exposures, but is not important for the study of temporary deposition in the skin and subsequent penetration to the receptor. As the lag-times for most pesticides tested were more than 6 hours, it was not surprising that the maximal fluxes for these pesticides obtained in the 6-hours exposure experiments were generally lower than in the 24-hours exposure experiments, whereas pirimicarb with a short lag-time had comparable Kρ values in experiments with 6 hours as well as 24 hours. An important observation was that most of the applied dose of the two most hydrophilic pesticides (pirimicarb and dimethoat) was recovered in the donor wash after 6 h, which implies that preventive measures in the form of handwash will significantly reduce the skin penetration of these pesticides following occupational exposures. However, an equally important observation was that dermal penetration continues long after exposure has ended due to absorption of a temporarily deposited pesticide present in the skin compartment at the end of exposure.
Nielsen and Nielsen have also studied the penetration of methiocarb, paclobutrazol and pirimicarb and lag-times between 10 and 18 h were reported (Nielsen & Nielsen, 2000) A later study by the same group found lag-times significantly deviating from these earlier observations. The receptor fluid did, however, differ between the two studies. Thus, a more physiological receptor (5% BSA in a 0.9% NaCl aqueous solution) was used in the later study when compared with the use of 50% ethanol in the earlier study. These differences make direct comparisons difficult as ethanol is known to significantly affect the barrier characteristics of the skin membrane (Nielsen, 2000). In general, these observations are in agreement with other studies demonstrating the influence of the vehicle on dermal penetration characteristics (Singer & Tjeerdema, 1993;Baynes et al., 2002;Brand & Mueller, 2002).
As illustrated in Fig. 2, different combinations of flux and lag-time may cause identical amounts of a chemical to cross the dermal barrier at a certain point in time. Thus, the total amount of a chemical penetrating during a certain time period is not very informative on its own. This is especially relevant in relation to short-term exposures such as most occupational exposures, where exposure time will often be less than or close to the experimental lag-time. Under these circumstances, an exposure assessment based on measurement of blood concentration at the end of a work shift will ignore pesticide deposited in the skin and demonstrate zero absorption and thus underestimate the actual exposure. Although literature indicates that the systemic concentration of a number of pesticides tested did not increase after exposure was ended (Zendzian, 2003), the continued presence of pesticide in the blood compartment indicates continued absorption from the dermal reservoir and thus a continued rise in systemic dose. As a prolonged presence of a lower concentration of a toxicant may be equally important as a short exposure causing a high blood concentration, we suggest that data used for regulatory agencies should include steady-state flux (or Kρ) and lag-time as well as an estimation of the potential importance of the skin reservoir.
Version 1.0 May 2009, © Danish Environmental Protection Agency