Dermal absorption of pesticides - evaluation of variability and prevention
12 Prevention of dermal absorption
- 12.1 Dermal exposure to pesticides
- 12.2 Personal protective gear
- 12.3 Penetration of benzoic acid through gloves
- 12.4 Penetration of pesticides through gloves
- 12.5 Types of gloves
- 12.6 The use and re-use of gloves
Risk is defined as the possibility that a worker or the environment may be harmed in a particular process. The toxicity or hazard of the pesticide cannot be altered, but the risk can be handled through use of appropriate protective gear and proper management and application.
12.1 Dermal exposure to pesticides
Exposure to pesticides is seen through working with different plants, fruits, crops, flowers etc. The degrees of exposure vary from the frequent, specific use in limited areas like for example greenhouses to the less frequent use in large outdoor facilities. The different intensities may be exemplified by the fact that a study from 2001 showed that a typical Danish ornamental greenhouse is using pesticides or growth retardants more than 50 times a year in contrast to conventional Danish farmland only being treated 2 to 3 times a year (Andersen & Nielsen JB, 2001).
Work-related exposure to pesticides may happen throughout the production, mixing, and loading of strong commercial products, distribution and management of diluted pesticides, and through re-entry activities. The different jobs are characterized by causing a combination of instant exposure (splash etc.) to concentrated commercial products and continued exposures to lower concentrations by handling stems, leaves or soil after pesticide treatment. Studies have found that workers performing the farm task of thinning are more exposed to pesticides then e.g. workers harvesting or pruning (de Cock et al., 1998b;de Cock et al., 1998a;Simcox et al., 1999). One group found a higher level of pesticides in the house and vehicle dust of the workers thinning and a higher pesticide metabolite concentration was found in their children’s urine as an evidence of the take-home pesticide pathway (Coronado et al., 2004;Coronado et al., 2006).
Previous investigations have examined workplace protective practices of field- and greenhouse workers. Their use of gloves, long-sleeved shirts, and long pants (Vela-Acosta et al., 2002;Sjelborg P et al., 2008) but only limited investigations have assessed pesticide exposure among these groups of workers (Simcox et al., 1999;Fenske, 2005;Strong et al., 2004) and the prevention of work-to-home transmission of pesticides as described in a review by Fenske 2006 (Fenske et al., 2006).
12.1.1 Mixing and loading
When the sales products are delivered to the user, the concentration of the product is significantly greater than the concentration used for treatment of the plants. When mixing and loading the pesticide there is a present risk of being exposed to the concentrated pesticide. Often the kind of exposure is an instant exposure e.g. splashes. If a worker has areas of skin not covered by protective gear (gloves, etc.) an uncontrolled splash may cause unintended exposure to the skin - often the hands - and may cause a significant risk of toxicity. Exposure of the hands can be an important contributor to the total exposure of the skin and has been shown to account for between 50 and 90% of the total body exposure (Abbott et al., 1987;Archibald et al., 1995;Karr et al., 1992).
12.1.2 Distribution
The distribution of pesticides may engage automatic spraying, spraying from person-driven vehicles, hand-carried spraying, or watering systems. Distribution involves varying exposure times to varying concentrations of pesticides with varying toxicity. Though, the concentrations will not be expected to cause acute toxicity after an accidental short-term exposure, it is still important to focus on avoiding long-term dermal contact with pesticides. This means a necessity to use gloves at all times during spraying operations, which again asks for a certain degree of comfort if good compliance should be attained. When distributing the pesticides a considerably part of the body may be exposed besides the hands - even the respiratory system may be involved. In these cases, gloves will only be part of the preventive attempt to diminish body dose.
12.1.3 Re-entry
Exposure by re-entry happens when workers enter areas recently treated with pesticides. Leaves, stems and soil may have pesticide depositions and workers may be dermally exposed to these residues. How long the pesticide remains on the plant or soil and therefore is an occupational risk depends on chemical stability, stability against sunlight, and metabolism of the active ingredient. Compared to mixing, loading and distribution the concentrations at re-entry are lower but at times workers are exposed to the pesticides for an entire working day. The crop handling may require some dexterity and may be difficult to obtain with all glove types and materials. Moreover, the workers may not be aware that they are actually exposed. Therefore it is important to define re-entry intervals that allow the pesticide to wash off or degrade before the crop is handled again and also to use gloves whenever handling plants recently treated with pesticides.
12.2 Personal protective gear
Most important when managing pesticides, whether it is in the process of mixing, loading or distributing or it is handling the crop after pesticide treatment, is the use of protective gloves. The main problem that influences the choice and ultimately the use of gloves in a work situation is the discomfort of the gloves, but also the resistance, durability and penetration characteristics of the gloves play an important role in the safety procedure. Often comfort and resistance are not easy to combine in one type of gloves and if so the gloves are most likely very expensive and therefore not a priority especially in the developing countries. Preferably, people handling pesticides should always use the glove giving maximum protection. Since this glove is often not the most comfortable choice, a problem has arisen. To try and solve this problem it is important to have specific knowledge on the pesticides used and the degree of exposure the worker is subjected to.
12.2.1 Penetration characteristics of gloves
Penetration characteristics may be described as breakthrough time and penetration rate. To judge the quality of the glove the breakthrough time is one of the most used parameters. This parameter describes the time from the beginning of the exposure until the pesticide appears on the inside of the glove. The time range is from 15 min to more than 24 hours for different pesticides through different types of glove materials (Creely & Cherrie, 2001;Ehntholt et al., 1990;Guo et al., 2001;Krzeminska & Szczecinska, 2001;Moody & Ritter, 1990;Moody & Nadeau, 1994;Purdham et al., 2001;Schwope et al., 1992;Silkowski et al., 1984). Further, the resistance against penetration through a specific glove material will depend on pesticide materials (Creely & Cherrie, 2001;Ehntholt et al., 1990;Guo et al., 2001;Krzeminska & Szczecinska, 2001;Moody & Ritter, 1990;Moody & Nadeau, 1994;Purdham et al., 2001;Schwope et al., 1992;Silkowski et al., 1984)as well as formulation (Ehntholt et al., 1990;Harville & Que Hee, 1989;Moody & Nadeau, 1994;Nielsen & Andersen, 2001;Purdham et al., 2001;Silkowski et al., 1984).
The reasons why gloves fail to provide effective protection can be divided into four categories:
- Misuse
- Physical damage
- Degradation
- Permeation
In many situations, it will be a combination of these four reasons that leaves the user exposed to the chemical, often without realizing it (Packham, 2006).
The breakthrough time will not say anything about the amount of pesticide absorbed into the glove or the penetration rate after exposure, but it is still an important measurement since, as illustrated in Figure 22, two glove types may have identical penetration after a 6 hour period but very different breakthrough time and penetration rate. Thus, if the work time were 4 hours, glove 2 would definitely be preferred.
Essential knowledge when using disposable gloves is:
- Information on breakthrough time
- Know when to change gloves to avoid crossing the limit for breakthrough time
- If the gloves are used after breakthrough it is worth knowing the penetration rate
Information on penetration rate and reservoir within the glove material is of importance if gloves are used several times. (Nielsen JB, 2005b).
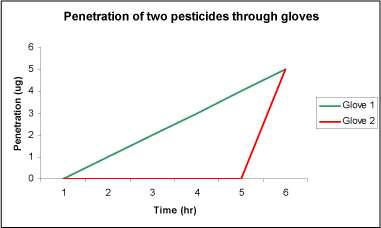
Figure 22: Penetration of two pesticides (1 and 2) through gloves. Penetration of 1 and 2 is identical, but the breakthrough times and penetration rate are different. (Nielsen JB, 2005b)
Another important parameter that may influence skin exposure is physical and/or chemical degradation of the gloves worn for personal protection. The influence of hand movements on the integrity of the gloves is often not considered when studying glove permeation/penetration. In a recent study a robotic hand, simulating normal hand motions, was used to assess the influence of hand movement on the permeation through nitrile rubber gloves. Even though hand movement did not appear to significantly affect the permeation of captan through the gloves, the movements did influence physical and/or chemical degradation, resulting in glove failure (Phalen & Hee, 2008). Thus, future research should continue to investigate the influence of hand movement and additional work factors on the permeation, penetration, and physical integrity of protective gloves.
12.3 Penetration of benzoic acid through gloves
Benzoic acid has been used as a model compound to demonstrate the effect of different glove materials used in an identical experimental set-up. Further, these studies allow important observations on the influence of dose on glove penetration. Eventually, these experiments demonstrate the significant fraction of the applied dose residing in the gloves when exposure is terminated, which is of considerable importance when discussing gloves intended for repeated use.
From figures 23 and 24 and Table 7 and Table 8 it is evident that gloves significantly reduces total penetration, but the effect depends on glove material, dose of chemical applied on the glove, and the influence on lag-time is limited. At the lowest dose, nitril offers very good protection as the maximal flux is reduced 25-fold as contrasted by the 50% reduction in penetration offered by the latex material (Table 7). The relative protection is drastically reduced at the higher dose (Table 8) and illustrates that a glove suitable for low dose exposure, e,g, during re-entry situations, may not be ideal for higher doses, e.g. during mixing and loading were undiluted pesticides are used. One explanation is that the glove functions as a temporary deposit, but with a limited capacity. Thus, substatntial amounts of benzoic acid are recovered from the glove material at the end of exposure, more so in nitril gloves (Tables 7 and 8). In experiments with gloves-only, comparable amounts of benzoic acid was deposited in the gloves as in experiments with underlying skin (Tables 7 and 9). Glove recovery accounted for 40% (latex) and 74% (nitril) of the applied dose of benzoic acid. In experiments with tebuconalzol, which is less hydrophilic and has a higher molecular weight, approximately 18% of the applied dose was recovred in latex gloves, which illustrates that glove deposition may vary depending on the pesticide as well as the dose applied.
Table 7. Dermal penetration characteristics following topical application of benzoic acid (4 mg/mL)
| Control | Latex | Nitril | |
| Cotton swabs (µg) | 15.6 | ||
| Donor wash (µg) | 11.6 | 11.3 | 12.9 |
| Donor recovery (µg) | 27.2 | 11.3 | 12.9 |
| Glove recovery (µg) | 116.3 | 271.1 | |
| Between glove and skin (µg) | 1.4 | 2.6 | |
| Epidermis (µg) | 1.3 | 4.2 | 3.2 |
| Dermis (µg) | 8.2 | 8.7 | 6.1 |
| Skin recovery (µg) | 9.5 | 12.9 | 9.3 |
| Receptor recovery (µg) | 398.4 | 239.5 | 42.1 |
| Experimental recovery (µg) | 435.0 | 381.3 | 338.1 |
| Experimental recovery (pct) | 102.6 | 89.9 | 79.7 |
| Maximal flux (µg/hr/cm2) | 13.1 | 6.6 | 0.5 |
| Lag-time (hr) | 2.5 | 3.1 | 7.0 |
Based on the maximal flux, an apparent Kρ may be calculated for the
penetration of benzoic acid in controls, and is found to be 33 µm/hr.
Table 8. Dermal penetration characteristics following topical application of benzoic acid (40 mg/mL)
| Control | Latex | Nitril | |
| Cotton swabs (µg) | 89.6 | ||
| Donor wash (µg) | 10.0 | 83.6 | 150.8 |
| Donor recovery (µg) | 99.6 | 83.6 | 150.8 |
| Glove recovery (µg) | 320.8 | 1225.6 | |
| Between glove and skin (µg) | 5.2 | 14.4 | |
| Epidermis (µg) | 8.3 | 10.8 | 17.1 |
| Dermis (µg) | 147.0 | 137.8 | 165.6 |
| Skin recovery (µg) | 155.4 | 148.6 | 182.8 |
| Receptor recovery (µg) | 4493.5 | 3815.6 | 2712.9 |
| Experimental recovery (µg) | 4748.5 | 4373.8 | 4286.4 |
| Experimental recovery (pct) | 112.0 | 103.6 | 101.1 |
| Maximal flux (µg/hr/cm2) | 166.4 | 104.0 | 47.4 |
| Lag-time (hr) | 1.2 | 2.4 | 2.4 |
Based on the maximal flux, an apparent Kρ may be calculated for the penetration of benzoic acid in controls, and is found to be 42 µm/hr.
Table 9. Penetration characteristics following application of benzoic acid (4 mg/mL) to static diffusion cells mounted with gloves only.
| Latex | Nitril | ||
| Donor recovery (µg) | 7.9 | 16.9 | |
| Glove recovery (µg) | 132.8 | 252.8 | |
| Glove recovery (pct) | 40.0 | 74.0 | |
| Receptor recovery (µg) | 190.1 | 72.6 | |
| Experimental recovery (µg) | 330.7 | 342.3 | |
| Experimental recovery (pct) | 78.0 | 80.7 | |
| Maximal flux (µg/hr/cm2) | 11.2 | 1.6 | |
| Lag-time (hr) | 0 | 1.8 |
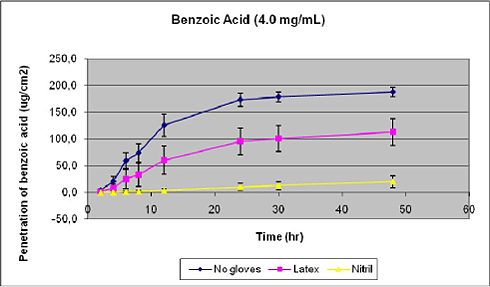
Figure 23. Influence of gloves on percutaneous penetration of benzoic acid. Glove material was mounted on top of the skin in the static diffusion cells. Results are given as mean + SEM (n=6).
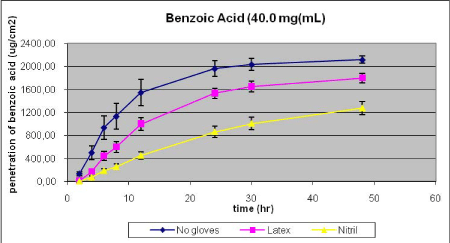
Figure 24. Influence of gloves on percutaneous penetration of benzoic acid. Gove material was mounted on top of the skin in the static diffusion cells. Results are given as mean + SEM (n=6).
Another interesting observation was that the observed lag-times in the experiments including human skin were not increased very much, which is in agreement with the low break-through times observed in the experiment with gloves only (Table 9). Lag-time for tebuconazol through latex gloves was comparable to the lag-time for benzoic acid through latex material (data not shown).
The results indicate that the amount of chemical residing between glove and skin is limited, irrespectively of glove material and dose (Tables 7 and 8).
The conclusions based on these experiments are that nitril gloves appear to offer a better protection than latex gloves against penetration of benzoic acid, but that the efficacy of the gloves is significantly reduced at higher doses. Single-use gloves should not be used for extended periods as lag-times are limited for both glove materials and an apparent accumulation occurs in the glove materials, which may reach saturation.
The observed accumulation of chemical within the glove material calls for further investigation as this may have profound influence on the guidance on use of gloves intended for repeated use. If a significant fraction of chemical is accumulated in the glove everytime it is used, the glove may become saturated and loose its efficacy for protection – without the user being aware of it.
Generally, the published information on penetration of pesticides through gloves is scattered and based on different experimental models mostly focusing on break-through times. Below we have summarized the available information on penetration of pesticides through gloves.
12.4 Penetration of pesticides through gloves
12.4.1 Carbamates
Penetration characteristics for these insecticides through gloves are studied for carbaryl, methomyl, sulfallate, methiocarb and pirimicarb (DuPont Co Protective Apparel Fabrics of TYVEK, 1993;Keith LH et al., 1000;Moody & Ritter, 1990;Nielsen & Andersen, 2001;Raheel & Dai, 1997;Raheel & Dai, 2002). The breakthrough times in all studies exceed 8 hours for PVC (polyvinyl chloride), nitrile butyl rubber, natural rubber and Neoprene® gloves (Moody & Ritter, 1990). Also gloves made of butyl rubber, nitrile or Viton® showed breakthrough times against sulfallate exceeding 8 hours, whereas Neoprene® had a breakthrough time of 4 hours only (Keith LH et al., 1000). Conclusions have been drawn that nitrile, butyl and Viton® have a higher resistance towards chemicals and penetration than latex/natural rubber or PVC gloves (Raheel & Dai, 1997;Raheel & Dai, 2002). Polyethylene gloves were penetrated by methomyl within 15 min (DuPont Co Protective Apparel Fabrics of TYVEK, 1993). Comparing the penetration of pirimicarb and methiocarb through latex or nitrile gloves has revealed that while latex reduces penetration of both carbamates by 50%, nitrile offers more than 90% protection against pirimicarb during an 18-hours test period (Nielsen & Andersen, 2001). In general, gloves made of butyl rubber, nitrile and Viton® proved to offer the best protection against carbamates.
12.4.2 Pyrethroids
Cypermethrin, permethin and tefluthrin were tested in this group of insecticides. Tefluthrin had a breakthrough time above 24 hours through nitrile, Neoprene and barrier laminate (SilverShieldTM trademark of Siebe North, Inc.)(Guo et al., 2001), but an increasing amount of pesticides remained attached to or absorbed into the glove made of barrier laminate by increased exposure time. Therefore barrier laminate gloves should not be re-used since they cannot be cleaned. Butyl rubber had a breakthrough time above 8 hours for cypermethrin (Krzeminska & Szczecinska, 2001) . Comparing the potential exposure (on the outside of the gloves) with the actual exposure (on the inside of the gloves) of permethin and two nitrile and one PVC glove it showed protection factors of 470, 200 and 96, respectively. This means that during a 20 min work period the penetration of permethin through gloves worn by volunteers was reduced to between 0.2% (nitrile) and 1% (PVC) of the potential exposure (Creely & Cherrie, 2001).
In general, the gloves made of nitrile, Neoprene, barrier laminate, butyl rubber and PVC showed good protection against penetration of this group of pyrethroids, when taking into account that barrier laminate is suggested to be used only as a single-use material (Guo et al., 2002).
12.4.3 Aryloxyalcanoic acids
2,4-D and MCPA were in this group of herbicides studies in different formulations. MCPA was tested in two undiluted formulations, one salt and one ester on four types of gloves (Purdham et al., 2001). The salt formulation showed no permeation in 24 hours but the ester formulation penetrated all four types of gloves within 24 hours - latex and Neoprene in 15 min and nitrile in 24 hours. The nitrile glove had the longest breakthrough time and the slowest penetration rate where Neoprene had a penetration rate that exceeded the other gloves by 3- to 7-fold (Purdham et al., 2001).
2,4-D demonstrated a breakthrough time above 8 hours in nitrile, butyl rubber, PVC, latex and Neoprene gloves (Krzeminska & Szczecinska, 2001;Moody & Ritter, 1990), although one study points out that Neoprene gloves were permeated much more than nitrile gloves (Harville & Que Hee, 1989). Several studies show that glove penetration varies between nitrile gloves and varies between different pesticide formulations and draws attention to the problems when extrapolating characteristics between pesticide formulations and even between gloves made of identical materials (Harville & Que Hee, 1989;Krzeminska & Szczecinska, 2001;Moody & Nadeau, 1994).
In general, the glove materials show fine protection against these herbicides, although gloves made of nitrile offered the longest breakthrough time and the lowest penetration rate after breakthrough.
12.4.4 Organochlorines
This group of insecticides have been analysed in two different studies (Ehntholt et al., 1990;Moody & Nadeau, 1994). Moody et al. demonstrated that DDT had an insignificant penetration through nitrile butyl rubber gloves during 24 hours (Moody & Nadeau, 1994), while Ehntholt et al. showed a breakthrough time of only 15 min for endosulfan (endosulfan 34% in xylene 57%) through several glove materials (butyl rubber, latex, polyethylene, PVC, SilverShield, nitrile) except for Neoprene which had a 30 – 60 min breakthrough time. The quantity of endosulfan penetrating the gloves during 8 hours demonstrated that 10 to 100 times less endosulfan penetrated gloves made of SilverShield and nitrile than other glove materials (Ehntholt et al., 1990).The conclusion was that gloves made of nitrile and SilverShield were most resistant to penetration and latex and polyethylene gloves the least resistant.
In general, the literature is scarce but suggests that breakthrough times for these insecticides are short in almost all types of materials, but SilverShield and nitrile gloves are the most resistant.
12.4.5 Organophosphates
Data on penetration characteristics through gloves of 8 insecticides in this group were identified. The pesticides were: azinphos-methyl, diazinon, ethyl-parathion, malathion, methyl-parathion, monocrotophos, tricresyl phosphate and terbufos (Ansell Protective Products, 1998;Ehntholt et al., 1990;Guo et al., 2002;Keeble et al., 1993;Keeble et al., 1996;Moody & Nadeau, 1994;Safety 4, 1993). Nitrile, Neoprene and SilverShield had a breakthrough time of more than 24 hours, although increasing exposure time showed that an increasing amount of pesticide was absorbed into the Neoprene gloves (Guo et al., 2001). Polyethylene gloves as well as cotton gloves reduced the penetration of azinphos-methyl and malathion in an in vitro study, but did not avoid penetration during a 4-hour observation period (Keeble et al., 1993;Keeble et al., 1996). When testing 4HTM laminate gloves from Safety4 a/s the breakthrough time increased to above 4 hours for malathion and glyphosate (Round Up®) (Safety 4, 1993). Gloves made of polyester or nylon showed no significant protection against azinphos-methyl (Keeble et al., 1993). The pesticide diazinon was tested in a sales formulation which demonstrated no significant penetration through nitrile butyl rubber over a 24-hour period. In table 10 different data on breakthrough times and relative permeation are summarized by Nielsen JB (Nielsen, 2005b).
Table 10. Breakthrough times and relative permeation of three organophosphates through seven glove materials
| Ethyl parathion | Methyl parathion | Azinphos-methyl | Monocroto phos |
Tricresyl phosphate | ||||||
| BT* (h) | RP* | BT (h) | RP | BT (h) | RP | BT (h) | RP | BT (h) | RP | |
| Natural rubber | 1-2 | + | <½ | +++ | <3 | + | 4-6 | ++ | <1 | + |
| Nitrile | 6-8 | 0 | 1-2 | ++ | >8 | 0 | 1-2 | ++ | >6 | 0 |
| Neoprene® | 3-4 | + | 1-2 | +++ | >8 | 0 | 3-4 | + | <½ | +++ |
| Polyethylene | <½ | +++ | <½ | +++ | ||||||
| PVC | 4-6 | + | <½ | +++ | 4-5 | ++ | <½ | ++ | >6 | + |
| Butyl rubber | >8 | 0 | 3-4 | ++ | ||||||
| SilverShield® | 4-6 | + | <½ | ++ | <½ | + | ||||
BT – breakthrough time; RP. – relative permeation
From this table it is shown that none of the glove materials perform very well against monocrotophos, though Neoprene and SilverShield have the lowest relative penetration. Similarly, methyl parathion penetrates most glove materials very fast and has a large quantitative penetration. Against the permeation of ethyl parathion, nitrile, SilverShield, PVC and butyl rubber demonstrated breakthrough times around 4-8 hours with butyl rubber and nitrile as the best material when relative permeation was taken into account. Not many of the tested glove materials demonstrated good penetration characteristics against tricresyl phosphate except for PVC and nitrile with a breakthrough time above 6 hours (Table 22.1).
All the current data illustrate the variety in penetration characteristics present even in the same group of pesticides. In general nitrile, butyl rubber and SilverShield were the most resistant towards penetration of the organophosphates that were tested. Latex, polyethylene and cotton were the least resistant. However, none of the glove materials demonstrated very good protection against all the pesticides studied.
12.5 Types of gloves
12.5.1 Polyethylene gloves
Different studies for the permeation of organophosphates, carbamates and organochlorines through polyethylene gloves were summarized and all data showed a very short breakthrough time of less than 15 min and a large penetration rate (DuPont Co Protective Apparel Fabrics of TYVEK, 1993;Ehntholt et al., 1990;Schwope et al., 1992)). Consequently, these gloves cannot be recommended as protection against pesticide exposure.
12.5.2 PVC
Only a few of the studied pesticides: 2,4-D, tricresyl phosphate and pentachlorophenol had breakthrough times above 6 hours (Ansell Protective Products, 1998;Moody & Ritter, 1990;Silkowski et al., 1984)). All other studied pesticides had a low breakthrough time and/or a high penetration rate and PVC generally offered poor protection against these pesticides (Creely & Cherrie, 2001;Ehntholt et al., 1990;Raheel & Dai, 1997;Raheel & Dai, 2002;Schwope et al., 1992).
12.5.3 Neoprene
Neoprene demonstrated good protection against some pesticides like: a carbamate, a triazine, several organophosphates, a pyrethroid and two aryloxyalcanoic acids (Ansell Protective Products, 1998;Cessna & Grover, 2002;Guo et al., 2001;Harville & Que Hee, 1989;Moody & Ritter, 1990;Purdham et al., 2001;Raheel & Dai, 1997;Raheel & Dai, 2002). But against organophosphates like ethyl-parathion and methyl-parathion, monocrotophos, endosulfan and pentachlorophenol the protection was poor.
12.5.4 Latex, natural rubber
Latex gloves have only shown breakthrough times of more than 8 hours in one study by Moody and Ritter when they studied carbamate carbonyl and 2,4-D. In this study the carbamate carbonyl was used as a dry powder, which may have influenced the penetration characteristics. All other data on this material for most pesticide groups show breakthrough times around 30 min and large penetration rates after that. A Danish field study on greenhouse workers demonstrated a protection by latex gloves of 93%, a breakthrough time for the fungicide Amistar® of less than 2 hours and an exposure of 3% when removing the gloves afterwards (Kirknel E & Sjelborg P, 2003).
If alternatives exist latex/natural rubber gloves should not be used as protective gear against pesticides.
12.5.5 Nitrile
All but one study demonstrate that nitrile gloves offer good protection against the pesticides tested (Ansell Protective Products, 1998;Creely & Cherrie, 2001;Ehntholt et al., 1990;Guo et al., 2001;Harville & Que Hee, 1989;Kirknel E & Sjelborg P, 2003;Moody & Ritter, 1990;Moody & Nadeau, 1994;Nielsen & Andersen, 2001;Purdham et al., 2001;Raheel & Dai, 1997;Schwope et al., 1992;Silkowski et al., 1984)). In most studies the breakthrough time was above 8 hours although one study showed breakthrough times for methyl-parathion, endosulfan and monocrotophos through nitrile glove of less than 30 min. In this study the conclusion was still that nitrile showed good protection to these pesticides compared with other gloves (Ehntholt et al., 1990). Kirknel et al. demonstrated a protection of 97% but also in this study exposure of the bare hands after removing the gloves was observed. This study group also demonstrated tears in the gloves at the end of experiments. In 17 out of 114 latex gloves this was the case whereas only 6 nitrile gloves were damaged (Kirknel E & Sjelborg P, 2003).
12,5.6 SilverShield/Laminate
Five pesticides have been tested against laminate glove material. The material showed relatively good protection against the pesticides: terbufos, tefluthrin, pentachlorophenol, 2,4-D and ethyl-parathion. (Ehntholt et al., 1990;Guo et al., 2001;Guo et al., 2002;Harville & Que Hee, 1989;Schwope et al., 1992). In one study monocrotophos, endosulfan and methyl-parathion penetrated the SilverShield gloves in 30 min (Ehntholt et al., 1990) and Guo et al. concluded that the laminate glove was a single-use glove given that the material could not be cleaned after use, but no penetration of the remaining pesticide was demonstrated (Guo et al., 2002).
12.5.7 Cotton liners
To increase the comfort of wearing gloves during work, cotton knit gloves (liners) worn under nitrile chemical-resistant gloves (CRG) have been tested on greenhouse workers. The workers felt more comfortable with the liners underneath their work gloves. However, contamination of the liners were demonstrated even though the degree of contamination was significantly lower than on the CRG (Stone et al., 2005). These results support the Environmental Protection Agency's recommendation that liners should be disposable (US Environmental Protection Agency, 2004).
12.6 The use and re-use of gloves
When using gloves as protective equipment against pesticide exposure it is important to know how to store and keep the gloves before and after use - if the gloves are reusable. As most people know from experience the structural integrity of glove materials decreases with time, but information on how the penetration characteristics change is insufficient and often information on storage conditions of the gloves is not available for the user. Raheel and Dai have demonstrated that only 10 days storage at -3°C changes the flexibility (nitrile gloves) as well as the resistance (Neoprene, nitrile and latex gloves) (Raheel & Dai, 2002).
12.6.1 Reusable gloves
Reusable gloves are often used around pesticides and other toxic substances. Repeated use without effective decontamination may result in secondary exposure. A study testing neoprene, Guardian butyl rubber, and nitrile synthetic rubber gloves against toluene and acetone used thermal decontamination and found this method to be effective in removing the solvents without significant degradation of the glove materials (Gao et al., 2005). Although the manufacturers of the gloves describe how to clean them before reuse, one study has shown that residues of pesticides still remain inside the gloves after cleaning (Guo et al., 2002). The same study suggested that gloves made of barrier laminate should not be reused since the material cannot be cleaned (Guo et al., 2002). Moody and Nadeau demonstrated a reservoir effect of 2,4-D, DDT and diazinon within the glove when a considerable amount of the substances could be extracted from the gloves after cleaning (Moody & Nadeau, 1994). As mentioned earlier, however, the study did not demonstrate if the pesticides were available for later penetration and absorption.
When protective gloves are taken off, exposure can be even more substantial and this is often underestimated (Garrod et al., 2001;Edwards et al., 2007). It is difficult to avoid touching the exterior of the gloves and a recent study has also shown that the inside of the gloves is often contaminated just as the outside (Creely & Cherrie, 2001;Garrod et al., 2001;Machera et al., 2003). If the hands are contaminated inside the gloves an occlusive environment and an intimate contact with the hazardous substance occur. Since occlusion has proven to increase skin penetration it is of great importance to avoid chemicals inside a glove (Wester & Maibach, 1983). More research on this issue is required to obtain further knowledge on how the occlusion affects the penetration. A few studies have studied gloves on top of a skin membrane (Keeble et al., 1996;Nielsen & Andersen, 2001), but data describing enhanced penetration through human skin are not further elaborated.
Kirknel and Sjelborg found that as much as 50% of the total pesticide exposure occurs when the employees change their gloves during work (Kirknel E & Sjelborg P, 2003). Because of the interindividual variation of exposure in this procedure, is it important that the users are aware of the risks and well educated to avoid unnecessary contamination. Rawson et al. showed that without training 9 out of 10 volunteers had internal contamination of their gloves when they reused them. However, if they were trained this was reduced to 1 out of 10. Although single-use gloves may generally reduce the potential for internal contamination, this study demonstrated that 3 out of 10 volunteers were contaminated due to leaking or faulty gloves. Wearing gloves which are internally contaminated can lead to increased systemic absorption due to increased area of contact and reduced skin barrier properties, and repeated skin contact with low volatility chemicals can give higher than expected exposure if evaporation of the carrier occurs and the concentration in contact with the skin increases (Rawson et al., 2005).
From practical experience it is also known that disposable gloves are sometimes reused. Since the integrity of the gloves changes and the gloves might be contaminated on the inside after prior use, the exposal situation becomes even more essential. When an employee actually falsely believes that the gloves protect against chemical exposure, the situation is worse than if a worker despite understanding the danger chooses not to use gloves since he is probably aware of the potential risk and therefore takes better precautions. A situation with an employee feeling safe, and acting as if protected, is unacceptable.
Because of the problems mentioned above, the important elements in the preventive strategy to reduce exposure must be to educate, train and supervise the users better. If the problem about reusing gloves (reusable and disposable) is not given further research attention the advice will be to only use disposable gloves and dispose them afterwards.
By using gloves and other personal protective equipments the hope is to change unacceptable exposure into an acceptable risk.
Version 1.0 May 2009, © Danish Environmental Protection Agency