Environmental Project No. 1292, 2009
Development and use of screening methods to determine chromium (VI) and brominated flame retardants in electrical and electronic equipment
Contents
- 1.1 Executive Order on RoHS
- 1.2 Homogeneous materials and passivation layers
- 1.3 Probability considerations in relation to the selection of samples
- 1.4 Screening using X-ray fluorescence spectrometry (XRF)
2 Presence of PBDEs and PBBs in electrical and electronic equipment
- 2.1 What are PBDEs and PBBs?
- 2.2 Applications of the substances in electrical and electronic products
- 2.3 Decision tree
3 Presence of hexavalent chromium in electronic equipment
- 3.1 What is hexavalent chromium?
- 3.2 Use of hexavalent chromium for anticorrosive coatings
- 3.3 Uses of metallic chromium
- 3.4 Chromium(VI) in plastic and paint/enamel pigments
- 3.5 Chromium in glass
- 3.6 Exemptions from the RoHS Directive
- 3.7 Decision tree
4 Screening method to determine hexavalent chromium in surfaces
5 Laboratory methods to determine Cr(VI), PBDEs and PBBs
- 5.1 Determination of PBDEs and PBBs in polymers
- 5.2 Determination of hexavalent chromium in surfaces
- 5.3 Colometric determination of chromium(VI) in materials
Foreword
The RoHS Directive
In order to limit the long-term human and environmental impact of hazardous substances, the EU has adopted the RoHS Directive which prohibits the marketing of electrical and electronic products containing more than 0.1% lead, mercury, hexavalent chromium, polybrominated biphenyls (PBBs) and polybrominated diphenyl ethers (PBDEs) by weight or more than 0.01% cadmium by weight. In this connection the substances comprised are collectively referred to as "RoHS substances".
So far the Directive does not comprise medical equipment, measuring and control equipment and major industrial equipment, and a large number of exemptions have furthermore been granted for applications where substitution is impracticable, or where the negative environmental or health impacts caused by substitution are likely to outweigh the benefits thereof. However, on 16 December 2008, the Commission submitted a proposal to the member states for a recast of the Directive to include medical equipment as well as measuring and control equipment.
The RoHS Directive entered into force on 1 July 2006, and in Denmark the Directive has been implemented by the Executive Order on the restriction of import and sale of electrical and electronic equipment containing certain hazardous substances, Executive Order No. 873 of 11/08/2006.
The RoHS substances have traditionally been used for a large number of purposes, and as electrical and electronic equipment is made up of multiple components, each of which is subject to the RoHS requirements, determining whether a product is compliant with the provisions of the Directive is an extensive task.
The content of lead, cadmium and mercury in individual materials can be determined easily and non-destructively using an X-ray Fluorescence Spectrometry (XRF) screening method. For hexavalent chromium and the two groups of brominated flame retardants, PBDEs and PBBs, the XRF method is not immediately applicable, and there has consequently been a need for the development of relatively simple and cheap screening methods.
Development of a screening method for hexavalent chromium
As part of the drawing up of this guidance, a simple screening method has been developed that can be used to get an indication of whether the products contain hexavalent chromium. The accuracy and reliability of the method has been reviewed in connection with the development work, and this forms the basis of the description of the method in this guidance.
Development of screening method for PBDEs and PBBs
We have also attempted to develop a screening method for PBDEs and PBBs based on thin-layer chromatography. The development work is described in Annex 1 to this guidance. We have succeeded in developing a screening method to determine PBDEs in ABS plastic using thin-layer chromatography. For other types of plastic, extraction of the substances from the plastic materials is very difficult and requires methods that are not suitable for use outside analysis laboratories. As a result, the screening method developed will only cover a small part of the PBDE applications in electrical and electronic products. Due to the limited field of application, the assessment is that the method is not feasible as a general screening method.
Purpose of this guidance
The overall purpose of this guidance is to help importers, producers and control authorities in their inspections of electrical and electronic products by describing the most likely occurrences of the substances as well as low-cost screening methods to determine hexavalent chromium.
Contractors
The screening methods and guidance have been drawn up by the Danish Technological Institute and COWI A/S and are subsidised under the Enterprise Scheme of the Ministry of the Environment.
Reservations
The screening methods described can only be used for indication purposes and cannot replace actual laboratory analyses performed according to current standards. In cases where the screening analysis results may give rise to third-party economic costs, verification by an accredited laboratory is therefore recommended. Information on the applications of chromium(VI), PBDEs and PBBs in electrical products reflect the knowledge available to the authors at the time of writing, but the possibility that some applications of the substances have not been included in the description cannot be excluded.
1 Introduction
- 1.1 Executive Order on RoHS
- 1.2 Homogeneous materials and passivation layers
- 1.3 Probability considerations in relation to the selection of samples
- 1.4 Screening using X-ray fluorescence spectrometry (XRF)
1.1 Executive Order on RoHS
In Denmark the RoHS Directive has been implemented by the Statutory Order on the restriction of import and sale of electrical and electronic equipment containing certain hazardous substances, Executive Order No. 873 of 11/08/2006.
Pursuant to the Executive Order, import and sale of electrical and electronic equipment containing more than 0.1% lead, mercury, hexavalent chromium, polybrominated biphenyls (PBBs) or polybrominated diphenyl ethers (PBDEs) by weight in homogeneous materials and more than 0.01% cadmium by weight in homogeneous materials has been prohibited as from 1 July 2006.
For a period of time the brominated flame retardant deca-BDE was subject to an exemption, but the exemption expired on 30 June 2008¹.
1.2 Homogeneous materials and passivation layers
Neither the RoHS Directive nor the Danish Executive Order defines what is meant by "homogeneous materials".
According to the EU Commission's interpretation as seen on its website, "homogeneous material" means a material which cannot be mechanically disjointed into different materials. Examples of homogeneous materials are individual types of plastic, ceramics, glass, alloys, paper, board, resins and coatings. Mechanical actions are stated to include unscrewing, cutting, crushing and abrasive processes. Examples mentioned on the Commission's website:
- A plastic cover is a "homogeneous material" if it consists of one type of plastic that is not coated with or has attached to it or inside it any other kinds of materials.
- An electric cable that consists of metal wires surrounded by non-metallic insulation materials is an example of a "non-homogeneous material" because the different materials could be separated by mechanical processes. In this case the 0.1% by weight limit value of the Directive would apply to each of the separated materials individually.
- A semi-conductor package contains many homogeneous materials which include: plastic moulding material, tin-electroplating coatings on the lead frame, the lead frame alloy and gold-bonding wires.
The uncertainty as regards the definition of homogeneous materials applies particularly to thin coatings, e.g. passivation layers on zinc, aluminium, copper and stainless steel.
For example, a thin passivation layer on a thin coating of zinc may contain hexavalent chromium. In principle, it is possible to remove the hexavalent chromium surface by abrading it with fine-grade sandpaper, but in practice it will be difficult to obtain sufficient material using this method to analyse the concentration of hexavalent chromium in the homogeneous surface layer.
Normally, the passivation layer can be dissolved with a suitable chemical solution, and the weight loss of a test piece can then be determined. Analysis standards for determination describe how to determine the weight of the passivation layer for chromated surfaces on zinc and cadmium coatings and on aluminium. These methods determine the volume per surface unit, and they cannot be directly used to determine the concentration of chromium(VI) in the passivation layer.
It seems to be generally accepted that passivation layers with hexavalent chromium contain more than 0.1% in the thin surface layer that is generated either on top of an electrolytically precipitated zinc layer or generated through a chemical reaction with pieces of aluminium, copper and stainless steel. Such passivation layers have widely different thicknesses and thus widely different chromium(VI) release potential per unit area. Passivation layers on copper and stainless steel are so thin that they cannot be seen with the naked eye (0.01-0.1 µm), while passivation layers on zinc and aluminium are typically somewhat thicker (0.2-2.0 µm).
As a consequence, it may be difficult to accurately determine the concentration of hexavalent chromium in the surface. The volume is often stated as a total volume per cm² and not as a concentration. The concentration can then be calculated if the surface thickness is known, but this may also be difficult to determine in practice. In many cases it is necessary to base the determination on the general knowledge about the thickness of the layers and the concentration of the substances in the layer, and then, based on an analysis showing the presence of the substances in the surfaces, to infer that the product is not compliant with the requirements of the RoHS Directive.
Thus, the analysis of chromium(VI) in the surface does not establish directly that the concentration exceeds 0.1% in the passivation layer itself, or that the passivation layer can be regarded as a homogeneous material. At the time of writing (March 2009), no court of law has delivered a ruling as to the documentation needed to determine the presence of excessive concentrations of the substances in thin surface coatings.
1.3 Probability considerations in relation to the selection of samples
Electrical and electronic products are composed of a large number of components, each consisting of different parts made of different materials: plastic, metal, ceramic materials, glass, etc.
A complete analysis for RoHS substances in all electrical or electronic product parts would easily comprise hundreds of analyses and be very costly. In this connection it is important to distinguish between the procedures in relation to documentation and control. Documentation of RoHS compliance should include documentation of all individual parts and materials.
On the other hand, for control purposes it may be relevant to be able to select the parts in which the RoHS substances are most likely to be found.
This guidance includes instructions as to where looking for individual substances is most relevant, and points out the exceptions to the RoHS Directive that should be taken into account.
1.4 Screening using X-ray fluorescence spectrometry (XRF)
A widespread screening method used by the regulatory authorities in a number of countries is screening using X-ray fluorescence spectrometry (XRF). Handheld instruments are available on the market for elemental analysis of a sample in less than a minute. The handheld instruments typically have a 1 x 1 cm measuring window, so only larger parts can be analysed. Laboratories have more sensitive instruments with measuring windows of less than 1 x 1 mm, which can be used to scan more complex parts such as printed circuit boards.
The handheld instruments are useful for analyses for lead, cadmium and mercury in large plastic parts, ceramic materials, glass, metal alloys, etc. However, it should be noted that the measurements may be highly uncertain because they often have to be taken under less than ideal measuring conditions.
The instrument works by exposing the material to an X-ray beam. This causes the individual elements of the material to emit light with characteristic wavelengths, thereby making it possible to determine the elemental composition based on the intensity of the individual wavelengths of the emitted light. The "depth" of the measurement depends on the light dispersal in the material. In plastic materials, measurements taken using a handheld instrument typically provide a picture of the composition in the top millimetres, while measurements in metals reveal the composition in the top approx. 0.4 mm. Ideally, measurements can only be taken of homogeneous materials. In relation to inhomogeneous materials, e.g. with several coatings on top of each other, the measurements should be interpreted very cautiously.
In relation to the PBDE and PBB substance groups, which both contain bromine, the instrument can be used to measure the presence of bromine, but this method cannot be used to determine the type of brominated compound. Consequently, an XRF screening can be used as the first screening step, but a positive XRF screening should be supplemented with a laboratory test as described in chapter 5 to get an indication of whether the bromine is present in the form of PBDEs or PBBs.
In relation to hexavalent chromium, an XRF screening can only be used to analyse for chromium in the material, but it is not possible to determine whether the chromium is in the hexavalent form (see the detailed explanation in Chapter 3). The use of handheld XRF instruments is also limited in connection with chromium occurring in very thin coatings, because the instrument measures the average content of chromium in a layer that is considerably thicker than the coating of hexavalent chromium. Furthermore, ideal measuring conditions are difficult to obtain with a handheld instrument with a relatively large measuring window of about 1 x 1 cm if, for example, the measurement concerns a screw. Here, the readout may well indicate a chromium content in the material of less than 0.1%, although the actual content is much higher in the thin surface coating.
This guidance assumes that the users of the guidance have access to screening for the elemental composition using an XRF instrument.
[1] Executive Order No. 449 of 03/06/2008: "Executive Order amending the Executive Order on restriction of import and sale of electrical and electronic equipment containing certain hazardous substances".
2 Presence of PBDEs and PBBs in electrical and electronic equipment
- 2.1 What are PBDEs and PBBs?
- 2.2 Applications of the substances in electrical and electronic products
- 2.3 Decision tree
2.1 What are PBDEs and PBBs?
Polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyls (PBBs) are two groups of substances that are or have been used as flame retardants in plastic and textiles. In some countries, including the USA, PBDEs are also known as PBDOs.
Both groups of substances are characterised by consisting of two benzene rings with 1 - 10 bromine atoms attached.
The figure below shows a PBDE with 10 bromine atoms called deca-bromodiphenyl ether (deca-BDE). Greek numerals are used to designate the substances based on the number of bromine atoms. The most common types are tetra-BDEs (with 4 bromine atoms), penta-BDEs (with 5 bromine atoms), hexa-BDEs (6), hepta-BDEs (7), octa-BDEs (8), nona-BDEs (9) and deca-BDEs (10). Corresponding designations are used about PBBs with different numbers of bromine atoms.²
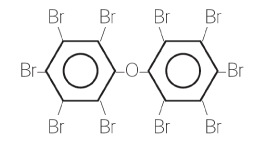
Commercial PBDE and PBB products normally consist of a mix of substances with different numbers of bromine atoms. The limit value of 0.1% applies to the sum of all PBDEs or the sum of all PBBs in the material.
Until recently, three commercial PBDE mixtures were marketed under the slightly confusing designations: penta-BDEs, octa-BDEs and deca-BDEs. The composition varies somewhat from one producer to the next, but the composition of products from Western producers has typically been in accordance with the below table.
| Commer-cial product | composition by percentage of a commercial product | |||||||
| tri- | tetra- | penta- | hexa- | hepta- | octa- | nona- | deca- | |
| Deca-BDE | 0.3-2 | 97-98 | ||||||
| Octa-BDE | 10-12 | 43-44 | 31-35 | 9-11 | 0-1 | |||
| Penta-BDE | 0-1 | 24-38 | 50-62 | 4-8 | ||||
PBDEs and PBBs are always used together with antimony trioxide (Sb2O3), which acts as a synergist, i.e. the presence of antimony trioxide increases the flame-retardant effect of PBDEs and PBBs. The bromine (Br)-antimony (Sb) ratio is typically about 3:1, but it may vary considerably.
2.2 Applications of the substances in electrical and electronic products
Presence of deca-BDEs
Deca-BDEs are the most commonly used PBDEs, and the world's total consumption amounted to more than 50,000 tons in about 2007. For a period of time, deca-BDEs were subject to an exemption from the RoHS Directive, but the exemption expired on 30 June 2008.
Deca-BDEs are produced by a number of companies. The most commonly used commercial products in Western countries are SAYTEX® 102E (Albemarle Corp.), Great Lakes DE-83 RTM (Great Lakes Corp.) and FR 1210 (ICL Industrial Products).
The table below states the most typical applications of deca-BDEs and the amount of deca-BDE and antimony trioxide (Sb2O3) the plastic should contain in order to comply with the highest fire safety standards, i.e. V-0 quality. Lower-level fire safety applications use smaller amounts of deca-BDE and antimony trioxide, which means that the cabinets of appliances will often contain smaller concentrations than stated in the table.
| Type of plastic | Typical applications in electrical and electronic products | Deca-BDE in V-0 quality plastic (percentage of plastic) | Antimony trioxide (percentage of plastic) |
| HIPS (high-impact polystyrene) | Cabinets for TV sets, mobile phones, AV equipment, remote controls | 12-13% | 4-5% |
| ABS | Cabinets and small moulded structural parts of many appliances | 13-15% | 5% |
| PBT/PET (thermoplastic polyester) | Switches, relays, plugs, connectors - components requiring a high level of durability | 10,4 % | 4% |
| Polyamide (PA, nylon) | Switches, relays, plugs, connectors - components requiring a high level of durability | 16-18 % | 6-7 % |
| Polyethylene and polypropylene (collectively often called polyolefins) | Cables and wires, film in capacitors, lamp sockets | 20-30% | 6-10% |
Until recently, the most common global application by far of deca-BDEs was in HIPS (High Impact Polystyrene) for back panels of TV sets for the American market. Deca-BDEs have generally not been used in TV back panels on the European market for many years. It is not possible to point out any obvious places to look for deca-BDEs, but in general deca-BDEs have not been used as flame retardants in printed circuit boards and casings for electronic components. For example, Deca-BDEs were used in about 10% of all flame-retardant plastic components of PBT/PET and polyamids, but in principle, they may be present in any plastic component; the application is mainly determined by the producers' attitude to using the substance.
In addition to the deliberate application, deca-BDEs may be present in plastic parts made of recycled plastic. In such parts, deca-BDEs may be present in lower concentrations with the addition of another brominated flame-retardant to obtain a sufficient flame-retardant level.
For several years, a number of major producers have announced that their products do not contain PBDEs and PBBs. PBDEs and PBBs are therefore not likely to be found products from those producers, which include: Dell, Hewlett-Packard Company (including HP and Compaq), Sony, IBM, Ericsson, Apple, Matsushita (including Panasonic), Intel and B&O.
Presence of octa-BDEs
Octa-BDEs have mainly been used for ABS-type plastic, which is used for cabinets and moulded structural parts in electrical and electronic products. Octa-BDEs used to be the preferred ABS flame retardant. ABS is used for many small parts where high-strength plastic is not required.
In about 2001, global consumption amounted to approx. 4000 tons. For several years, Octa-BDEs and penta-BDEs have been prohibited in all types of products sold in the EU, and production seems to have been more or less discontinued worldwide.
Octa-BDEs were typically used in concentrations of 12-18% with 4-7% antimony trioxide.
Consequently, it is not likely that octa-BDEs have been deliberately added to electrical and electronic products, but as ABS plastic is suitable for recycling, octa-BDEs may well be present in all parts manufactured of recycled ABS.
In cases where octa-BDEs have been present in part of the recycled plastic, small amounts of octa-BDEs may be present in the plastic with the addition of another brominated flame retardant to obtain a sufficient flame-retardant level. Small, moulded, dark grey or black parts will often be manufactured of recycled glass where the black pigment ensures a homogeneous appearance regardless of the recycled plastic parts used in the lot.
Presence of penta-BDEs
Penta-BDEs have mainly been used in foam products, and penta-BDEs are not very likely to be found in electrical and electronic products.
Presence of PBBs
PBBs have not been manufactured for a number of years, and deliberately added PBBs are not very likely to be found in new electrical and electronic products. The only type of PBB that has been in commercial use is deca-BB, small amounts of which were used in HIPS, PBT and PET in concentrations of about 10%. PBBs may be present in recycled plastic in lower concentrations with the addition of another brominated flame retardant to obtain a sufficient flame-retardant level.
Rule-of-thumb applying to all PBDEs and PBBs
PBDEs and PBBs are always used together with antimony trioxide, which acts as a synergist.
A number of brominated flame retardants are not used together with antimony, and the presence of antimony can therefore be used to rule out plastic with such flame retardants from further examination. Unfortunately, typical alternatives to PBDEs and PBBs are also used together with antimony trioxide, so it cannot be concluded that the presence of Br and Sb together indicates the presence of PBDEs or PBBs.
The following rule-of-thumb can be used to interpret the results of an XRF analysis:
- Br is not present > the plastic does not contain PBDEs or PBBs;
- Br is present, but Sb is not present > the plastic does not contain PBDEs or PBBs;
- Br and Sb are both present > the plastic may contain PBDEs or PBBs.
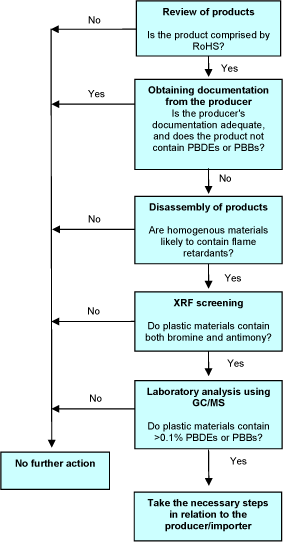
2.3 Decision tree
The decision tree below illustrates the procedure of the proposed method.
As regards documentation from producers of products or components, control may obviously be necessary even though the documentation seems to be adequate, but when planning the control measures, it may be relevant to give higher priority to products with less adequate documentation. Minimum documentation is a producer's certificate stating that the product does not contain RoHS substances above the limit value, but it is better if such a certificate is supported by an analysis report from an independent analysis laboratory. In this connection it should be noted that an analysis report may often be available which states the results of the analyses of a number of the product's components without all components having necessarily been analysed. It is therefore necessary to critically review the analysis report in terms of components that may contain RoHS substances.
Figure 1 Decision tree in relation to PBDEs and PBBs
[2] Apart from deca-BDE, which only exists in one form, there may be different isomers of each type - substances with the same number of bromine atoms, but with the bromine atoms in different positions. For example, there are 3 nona-BDE isomers and 12 octa-BDE isomers. In total, there may be 209 different PBDE combinations, in this connection referred to as 209 congeners. The term congeners is used about substances built around the same basic structure, but with a varying number of bromine atoms in different positions.
3 Presence of hexavalent chromium in electronic equipment
- 3.1 What is hexavalent chromium?
- 3.2 Use of hexavalent chromium for anticorrosive coatings
- 3.3 Uses of metallic chromium
- 3.4 Chromium(VI) in plastic and paint/enamel pigments
- 3.5 Chromium in glass
- 3.6 Exemptions from the RoHS Directive
- 3.7 Decision tree
3.1 What is hexavalent chromium?
Chromium occurs in both metallic form and as ions (chemical building blocks) in solutions or solid salts. Chromium ions may occur in three different forms known as oxidation states, which determine how the chromium ions react with other substances. The two most common oxidation states for chromium ions are 3 and 6, but occasionally, chromium ions may also be present in oxidation state 2. Chromium in oxidation state 6 is also called hexavalent chromium, because of the atom's ability to bond with six other atoms. It is also called chromium(VI) or Cr(VI). Correspondingly, the two other forms are called trivalent chromium, Cr(III), and divalent chromium, Cr(II), while metallic chromium in oxidation state 0 is often called Cr(0).
Hexavalent chromium is highly oxidising and the form that is most hazardous to the environment and to health. This is why the RoHS Directive only comprises hexavalent chromium. Normally, hexavalent chromium is not present in nature, and salts with hexavalent chromium are almost always man-made. Hexavalent chromium released from a product will relatively quickly react with other substances (e.g. iron(II)), thereby reducing the chromium to a lower oxidation state. When sampling, it should therefore be noted that a chemical reaction by which the hexavalent chromium is changed to the trivalent form is not allowed.
Hexavalent chromium will not be present in electrical and electronic products as a natural contamination element, so it is only necessary to check for hexavalent chromium where it can be deliberately used in the products.
Traditionally, the most common applications of hexavalent chromium in electrical and electronic equipment have been:
- Anticorrosive coatings on metal parts;
- Pigments in plastic parts and paint/enamel;
- Emerald green glass.
3.2 Use of hexavalent chromium for anticorrosive coatings
Passivation (also called chromating) with hexavalent chromium is used to protect metal surfaces against corrosion. The corrosion resistance of a large number of metals, including zinc, aluminium, cadmium, copper and stainless steel, can be strengthened by treatment with chromate-based solutions. The treatment generates a very thin surface consisting of chromium salts that make the metal part especially corrosion-resistant.
Passivation with hexavalent chromium is used in electrical and electronic equipment, particularly for coating of electro-galvanised steel and aluminium.
Metal parts coated with hexavalent chromium can be placed anywhere in appliances and individual components, e.g.:
- Screws, rivets, bolts;
- Frames and chassis;
- Electric switches, etc.;
- Plugs and terminators;
- Spacers;
- Antennae and accessories;
- Bars, etc.
Chromating also ensures better paint and enamel adhesion, and steel, aluminium and zinc parts are therefore often chromated before painting.
Electro-galvanised steel is coated with a thin layer of zinc (5-20 µm), traditionally topped by a thin layer of hexavalent chromium. Galvanised steel parts treated with hexavalent chromium have four typical appearances:
- Clear metallic with a slightly bluish tinge (thin layer; from 0.025 µm)
- Yellowish with a pearlescent sheen (medium-thick layer: 0.3-0.6 µm)
- Olive/dark colour (thickest layer; up to 1.5 µm)
- Black colour (< 1.5 µm)
The colours are familiar from DIY centres where the clear metallic screws are for indoor purposes and the coloured ones for outdoor purposes.
In Denmark, the use of hexavalent chromium for blue passivation of zinc has been largely discontinued for the last 10 years. Blue passivation can be attained with a chemical solution containing chromium(III) salts, and the passivation layer will therefore not contain chromium(VI). This probably applies to most Western European countries and the USA. On the other hand, it must be assumed that to a large extent chromium(VI)-containing chemicals are still used for blue passivation in Eastern Europe, Asia and Africa.
In Denmark and Western Europe, the trend is towards yellow and olive passivation without the use of chromium(VI). This trend was mainly a consequence of the RoHS Directive, and as a result, chromium(VI)-free zinc passivation is now also used in areas other than electrical and electronic components. Until recently, chromium(VI) was primarily used for yellow and olive passivation in Eastern Europe, Asia and Africa.
Typical contents of hexavalent chromium in coatings appear from the table below. In addition to hexavalent chromium, these coatings typically contain 70-90% trivalent chromium. In electrical and electronic equipment for indoor use, thin, clear coatings have been most commonly used. To some extent, yellow and olive coatings have been used in equipment for outdoor use or for use in corrosive environments. Black-chromated surfaces are primarily used for decorative purposes.
Table 1 Layer thickness, weight and chromium content of passivation layers on zinc and aluminium
| Passivation | Layer thickness µm |
Layer weight mg/m² |
% chromium in passivation layer |
% Cr(VI) of total chromium |
| Zinc, clear | < 0.2 | < 100 | 30 | 0 - 20 |
| Zinc, blue | < 0.2 | 50 - 500 | 35 | 0 - 20 |
| Zinc, yellow | 0.3 - 0.6 | 500 - 1500 | 35 - 40 | 20 - 30 |
| Zinc, olive | < 1.5 | > 2000 | 40 - 50 | 20 - 30 |
| Zinc, black | 2000 | 30 - 50 | no data | |
| Aluminium, clear | 50-100 | 30 | 20 - 25 | |
| Aluminium, yellow | 250 - 500 | 40 - 45 | 20 - 30 |
Largely all aluminium parts in electrical and electronic equipment have traditionally been coated with hexavalent chromium to avoid surface oxidation. This is often referred to as the Alodine process (Henkel) with regard to both yellow and bright passivation. Chromated aluminium surfaces are used inside electronic products. The corrosion resistance of bright passivated aluminium is more limited than that of yellow chromated aluminium.
Yellow chromating of aluminium is also used for pre-treatment of aluminium products before painting and enamel painting. This ensures good paint adhesion and extra strong corrosion resistance of the painted surface. A number of other passivation products without chromium have been introduced over the last 5-10 years and have gradually gained a foothold in the market because of the RoHS Directive and a general desire to minimise the use of chromium(VI).
Traditionally, hexavalent chromium has also been used for passivation of electrolytically deposited copper foils used in printed circuit boards, Li-ion batteries and plasma screens. The layer is typically less than 15 nm (0.015 µm) and the chromium content amounts to 0.02-0.03 mg/m².
Screening methods
In new RoHS-compliant equipment, hexavalent chromium is often replaced by a layer consisting exclusively of trivalent chromium, Cr(III), or the equipment is completely chromium-free. This means that an XRF instrument that is only capable of indicating whether chromium is present or not would not be able to give a good indication of whether the surface contains hexavalent chromium. In addition, a handheld XRF instrument readout would indicate a very small chromium content because of the thinness of the surface layer.
The screening method developed (section 4) can be used to examine whether hexavalent chromium is present in the surface of a metal part, and in principle the method can be used on all metal parts with metallic surfaces. For metal parts that have also been painted or enamel painted, it will be necessary to remove the paint/enamel first, thus rendering the screening method fairly uncertain.
None of the screening methods are suited for examining whether hexavalent chromium has been used to passivate the surface of electrolytically deposited copper foils.
3.3 Uses of metallic chromium
Chromium is widely used as an alloying element in steel. In these cases the chromium is always in the metallic form, Cr(0). Stainless steel typically contains 12-18% chromium, while other types of steel contain 0.1-2% chromium. Corrosion of the steel may generate chromium compounds, but they will not consist of hexavalent chromium.
The familiar bright, chromium-plated surfaces of steel, copper and brass consist of metallic chromium. In most cases, hexavalent chromium continues to be used to produce such chromium layers, but hexavalent chromium will not be present in the finished pieces, because the chromium(VI) salts are rinsed off after the electrolytic process. For several years it has been possible to produce these chromium coatings using chromium(III) salt electrolysis, and now the method finally seems to be gaining a foothold in Denmark. The traditional chromium-plating process using hexavalent chromium continues to be widely used, however.
3.4 Chromium(VI) in plastic and paint/enamel pigments
Hexavalent chromium is used in a number of pigments.
Together with lead and molybdenum, hexavalent chromium generates a number of pigments in clear red, orange, yellow and green colours. Lead chromates and lead-molybdenum chromates have traditionally been important pigment types in plastic and paint.
In electrical and electronic equipment these pigments will primarily occur in plastic of clear red, orange, yellow and green colours that may be found in:
- Cabinets;
- Plastic parts on individual components, including switches, fuses, etc.;
- Wire/cable insulation.
Together with strontium, barium or zinc, hexavalent chromium generates a number of pigments that have traditionally been used for anticorrosive paints. Such paints have typically not been used in electrical and electronic equipment, but the possibility that they may be used in equipment for outdoor use or for use in corrosive environments cannot be excluded.
Pigments with trivalent chromium yield olive-green colours, but such pigments are mainly used in other product areas, including cosmetics, soaps, detergents and paint.
Screening methods
As the clear red, yellow and green pigments are based on lead chromates and lead-molybdenum chromates and also contain lead, an XRF screening showing a lead content of more than 0.1% will be sufficient to determine whether a plastic part complies with the RoHS requirements. It should be noted that when measuring chromium in thin plastic parts, the XRF instrument readout may well indicate a chromium content of less than 0.1%, even though the actual content is higher.
The presence of strontium or barium together with chromium, determined with an XRF instrument, may indicate the presence of anticorrosive paints based on hexavalent chromium, but it has not been examined whether similar results would be obtained if the elements are present as alloying elements in steel.
The screening method developed (section 4) cannot be used to determine hexavalent chromium in plastic and paint pigments.
If any plastic parts contain chromium, but not lead, a laboratory analysis is necessary to determine whether the chromium is present in the hexavalent form.
3.5 Chromium in glass
Hexavalent chromium has traditionally been used to manufacture a particular type of green glass: clear emerald green crystal. It is stated that the chromium content may be up to 2%. This type of glass seems to have been used mainly for decorative purposes, and in relation to electrical and electronic products it is primarily mentioned in connection with crystal lamps and watches/clocks with electronic parts. An XRF scanning of green crystal showing chromium content would indicate the presence of hexavalent chromium, but a laboratory analysis is necessary to determine with certainty whether the chromium is present in the hexavalent form.
3.6 Exemptions from the RoHS Directive
The list of exemptions from the RoHS Directive includes two exemptions relating to hexavalent chromium. Both exemptions concern the use of hexavalent chromium for passivation in certain product groups.
The two exemptions concern:
- Hexavalent chromium used as an anti-corrosion of the carbon steel cooling system in absorption refrigerators;
- Hexavalent chromium used for corrosion preventive coatings of unpainted metal sheets and fasteners and used for corrosion protection and electromagnetic interference shielding IT and telecommunications equipment (the exemption expired on 1 July 2007).
3.7 Decision tree
The decision tree below illustrates the procedure of the proposed method in relation to hexavalent chromium used for coatings. Obviously, it is also possible to proceed straight to laboratory analyses without prior screenings. The decision tree shows two possible options in case of a positive screening. If the screening clearly indicates the presence of hexavalent chromium, it will be possible to contact the producer on this basis alone, but verification of the positive result by an accredited laboratory test is also an option. In any case, if the result is more doubtful, it is relevant to verify the result by a laboratory analysis.
Chapter 5 includes a particular decision tree for the screening stage.
For documentation from producers, see the corresponding section under PBDEs and PBBs.
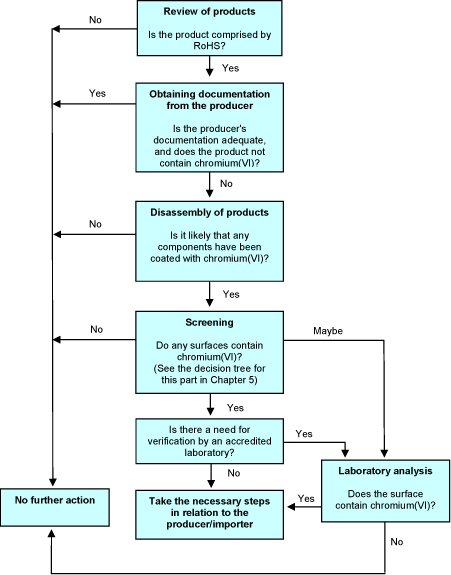
Figure 2 Decision tree in relation to metal surfaces containing hexavalent chromium
4 Screening method to determine hexavalent chromium in surfaces
In order to determine whether a piece contains chromium in a particular oxidation state (in this case hexavalent chromium, in the following referred to as chromium(VI)), it is necessary to use methods based on redox reactions that are specifically sensitive to the oxidation state in which chromium is present.
The International Electrotechnical Commission published an international standard in January 2009, including procedures for the determination of levels of regulated substances in electrotechnical products, IEC 62321.
This standard provides a method to determine levels of chromium(VI) (for further information about the method, see Chapter 5). The method is based on a colorimetric determination of the chromium(VI) content in a test liquid (colorimetry = determination by colour reaction). It is based on the reduction of chromium(VI) to chromium(III) in a reaction with 1.5 diphenylcarbazide oxidised to 1.5 diphenylcarbazone.

1.5 diphenylcarbazide
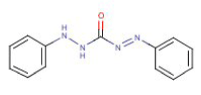
1.5 diphenylcarbazone
1.5 diphenylcarbazone yields a characteristic reddish purple colour with an absorption maximum of approx. 540 nm.
For a precise determination of the intensity of the red colour, and thus the chromium content in the test liquid, a spectrophotometer is required, but the red colour can also be observed with the naked eye, and the screening method developed is based on that principle.
The colorimetric method is also used to determine chromium(VI) in other media, and commercial test kits are available for drinking water and waste water analyses, among others, that are capable of testing for chromium(VI) in liquid volumes of 5-10 ml in special test tubes. An example is the Chromate Cell Test from Spectroquant®, which follows the US standards method 3500-Cr D. Spectroquant® test kits are available from VWR - Bie & Berntsen A/S, among others.
4.1 Development work
A number of experiments have been conducted in order to examine the possibilities and limitations of a screening test method based on visual colorimetric determination. The experiments, which are described in further detail in Annex 2, examine the sensitivity of the method in relation to different test materials. The test conditions most suited for a simple and quick screening method to be used without access to a fully equipped analysis laboratory have also been examined.
Based on the development work, the following section describes a test procedure and provides instructions as to the interpretation of test results.
4.2 Screening method procedure
The screening method can be used to determine chromium(VI) in passivation layers on metal pieces.
As mentioned above, the method is based on a colour reaction in a test liquid. In order to test for chromium(VI) in the passivation layer it is necessary to dissolve chromium(VI) in the test liquid and to ensure that chromium(VI) is not oxidised to chromium(III) before the colour reaction occurs.
Two methods are based on the corresponding methods described in IEC 62321.
- A spot test where chromium(VI) is dissolved in a drop of test liquid directly on the piece or in a white weighing boat.
- A screening boiling test where the piece is extracted for some time in de-ionised water at near-boiling point temperatures.
Neither test can be used to determine unambiguously whether the concentration in a homogeneous material is over 0.1%, but they can be used to detect the presence of chromium(VI). The screening boiling test can also be used to determine the concentration of chromium in a test liquid and thus yield a quantitative measurement. This can be used as an indirect indication of the concentration in the passivation layer under certain assumptions regarding the thickness of the passivation layer. It should be noted, however, that this also applies to the laboratory analyses described in IEC 62321. When testing for chromium(VI), the spot test should be performed first. If there is any doubt about the test result, it should be followed up with a screening boiling test.
As previously mentioned in section 1.4, it is possible to use an XRF instrument to determine whether there is chromium(VI) in a surface, but in practice, ideal measuring conditions can often not be obtained using a handheld instrument with a relatively large measuring window. Thus, the chromium content readout may well be under 0.1% even though the piece is coated with a thin chromium(VI) passivation layer. Proceeding directly to the spot test is therefore recommended.
4.2.1 Test solution
A diphenylcarbazide solution for colorimetric testing is produced by dissolving 0.4 g 1.5 diphenylcarbazide in a mixture of 20 ml acetone and 20 ml 96% ethanol, adding 20 ml 75% H3PO4 and 20 ml de-ionised water after dissolution. The solution should be left for eight hours before use.
The diphenylcarbazide solution is used for both test methods and will be referred to as the "test solution" in the following text.
Experience shows that for spot tests, using a diluted spot test liquid for certain surfaces is also expedient. Such liquid is produced by mixing spot test liquid and de-ionised water in the ratio 1:6. This is referred to as the "diluted test solution" in the following text.
It is important not to let the solution get too old as it discolours in the course of time. This may yield incorrect positive results, i.e. a colour reaction incorrectly indicating the presence of chromium(+VI). Discarding the test liquid when it is more than two months old is recommended if a strong coloration/precipitation of brown particles is observed, or if contamination of the liquid is suspected.
4.2.2 Spot test
Perform the spot test as follows:
- Degrease the piece with alcohol (or another suitable solvent) to remove fingerprints, dirt, etc. Let the solvent evaporate.
- If there is a layer of enamel: Using grain size 800 sandpaper, carefully abrade the part of the surface on which the test is to be conducted without exposing the underlying iron surfaces. If the underlying iron surfaces are exposed, the exposed iron may react with the chromium(VI) during the test, thus yielding a false negative result. Chemical cleaning agents are not to be used as they would cause a chemical reaction with the chromium(VI) layer. Tests have shown that it is difficult to abrade just the right amount, so sending pieces that are clearly enamel-painted for laboratory analysis should be considered.
- Place 1-5 drops of test solution on the surface of the piece. For small pieces such as screws it is recommended to perform the test in a white weighing boat as this makes it easier to see the colour of the liquid. It is also possible to perform the test in a small test tube, holding the tube up against a white background. The pictures below show a positive and negative test result, respectively.

Yellow chromated screw with test solution. The characteristic reddish purple colour indicates the presence of chromium(VI).

Chromium(VI)-free screw. No colour reaction is seen in a test solution or diluted test solution.
- Compare the colour with a blank sample where a few drops of test solution are placed in a weighing boat without a metal piece. Before beginning the test, make sure the weighing boats give no colour reaction. The reddish purple colour should occur within 1-2 minutes and be stronger than the colour of the blank sample. The colour will bleach relatively quickly and should be registered within 2 minutes.
- If the result is negative, or in case of effervescence: Repeat the spot test using diluted spot test liquid. Strong effervescence indicates that the acid reacts with the metal layer, the underlying metal possibly releasing e.g. iron(II). The iron may reduce the chromium(VI) to chromium(III), thereby yielding a false negative result.
This is not described in the standard, but experiments have shown that for pieces with blue or clear chromium(VI) passivation layers it is often easier to see the colour reaction when using a diluted test solution. The results are illustrated in the photos below.

Spot test of blue chromated screw with normal test solution. The lack of reaction is probably due to the relatively quick dissolution of the chromate layer by the phosphorous acid and the associated release of iron(II) ions or zinc(II) from the underlying material. These ions will react with chromium(VI) and reduce it.

Spot test of the same type of screw with diluted test solution.
- If the result remains negative: To ensure there is no clear, transparent enamel on the piece, carefully abrade the part of the surface on which the test is to be performed using grain size 800 without exposing the underlying iron surfaces.
- Repeat the spot test.
If the result remains negative, the surface layer is not likely to contain any chromium(VI).
Risk of false positive results
The experiments yielded no false positive results. Chromium(III)-coated surfaces gave no colour reaction. However, the method is unsuitable for red-enamel painted pieces that may release colour to the test solution.
Risk of false negative results
False negative results may be obtained if iron parts are exposed which may release iron that reacts with chromium(VI). Make sure the test solution is only in contact with intact surfaces (except deliberately abraded surfaces).
It may be difficult to abrade the surface to remove enamel, and for enamelled pieces there is a great risk of not exposing the relatively thin layer of chromium(VI) because in most cases the chromium layer will be much thinner than the enamel layer. Laboratories performing tests according to the standard will have similar problems abrading the surface, however.
The method is destructive
After testing, the piece will not show that it has been tested, but because part of the passivation layer is removed, the piece will subsequently be more exposed to corrosion.
4.2.3 Screening boiling test
IEC 62321 recommends proceeding with a boiling test to verify the result of the spot test if there is any uncertainty about it, i.e. primarily
- if the colour reaction of the spot test is so weak that it is difficult to distinguish from a negative result, or
- in case of extensive effervescence in both the spot test liquid and the diluted spot test liquid.
A positive spot test is very likely to yield a positive result in a boiling test and therefore requires no further verification.
According to IEC 62321, the test requires a surface area of 50 ±5 cm². It takes a substantial number of screws and other small pieces to make up a surface area of 50 cm², and getting a sufficient number of identical pieces from one piece of electrical and electronic equipment will often not be possible. Furthermore, a boiling test requires the piece to boil for some time, which may be difficult in a screening test.
As a result, a screening test requiring less test liquid has been developed, as experiments have shown that the test can be performed on a surface of as little as 7-10 cm² and using a simpler extraction method. Experiments have shown that this method yields a satisfactory result when an exact quantification of chromium(VI) in the test liquid is not required. If sufficient material (50 ±5 cm²) and access to laboratory facilities are available, use of the standardised method described in IEC 62321 is recommended.
According to experiments, the method does not work if the piece contains exposed surfaces such as the fractured surface of a broken screw. This is probably due the iron released from the fractured surface reacting with chromium(VI). The same applies to tests performed in accordance with IEC 62321.
- Determine the surface of the pieces (using suitable geometric formulas) and select a number of pieces with a total surface of approx. 10 cm².
- Degrease the pieces with alcohol (or another suitable solvent) to remove fingerprints, dirt, etc. Let the solvent evaporate.
- If there is a layer of enamel: Using grain size 800 sandpaper, carefully abrade the part of the surface on which the test is to be performed without exposing the underlying iron surfaces. If the underlying iron surfaces are exposed, the exposed iron may react with the chromium(VI) during the test, thus yielding a false negative result. Chemical cleaning agents are not to be used as they would cause a chemical reaction with the chromium(VI) layer. Tests have shown that it is difficult to abrade just the right amount, and sending pieces that are clearly enamel-painted for laboratory analysis should be considered.
- Place the piece in a test tube with the following dimensions: internal diameter 14 mm, external diameter 16 mm, height 100 mm. Pour 10 ml de-ionised water into the test tube. Measure the distance to the edge of the tube.
- Place the test tube in a wire mesh holder, e.g. with white polycoating, a hole diameter of 18 mm/height 60 mm and 3*3 holes making it possible to test nine samples at the same time.
- Place the piece(s) in the test tube(s), transfer the wire mesh holder to a pot with boiling water reaching the liquid level in the tubes. Use a pair of tongs to transfer the holder. Extract for 10 minutes. Place a glass lid on the pot after making sure that chromium(+VI) is not released by any metal pieces (screws), etc. on the glass when spot testing the metal pieces.
- Remove the test tube from the pot and refill with liquid to distance a from the edge of the tube.
- Remove the piece from the tube and cool down the liquid to room temperature.
- Add 0.2 ml 75% phosphorous acid to the test tube, shake and distribute the liquid in two test tubes containing 5 ml each.
- Add 0.2 ml test solution to one test tube and shake. No test solution should be added to the other test tube, which acts as a blank sample.
- Compare the colour of the liquids in the two test tubes. If the test tube containing the test solution is clearly redder than the blank sample, the piece contains chromium(+VI).
The result can be compared with the colour scale shown in Figure 3. If the result matches the colour of 0.2 ppm (mg/L) or more, the piece can be regarded as containing chromium(+VI) exceeding the limit value (unless the blank sample is also coloured). Multiply the limit of detection to be seen with the naked eye of approx. 0.1 mg/L by an uncertainty factor 2 to make the determination fairly certain. If the colour is below that level, a laboratory spectrophotometer test is required to obtain a more certain determination.
The test liquid concentration can be converted to chromium(VI) per m² surface. If the pieces have a total surface of 10 cm², 0.2 ppm in the test liquid will correspond to approx. 2 mg chromium(VI)/m², if all the chromium(VI) has been dissolved in the test liquid. The screening test gives only a rough indication of the amount of chromium(VI) per m². If a more exact determination is required, a laboratory analysis in accordance with the standard is necessary.

Figure 3 Colour scale - liquids for calibration: Concentrations in ppm from left: 0.05/0.1/0.2/0.5/1/2.
Neither the screening boiling test nor the laboratory analyses can be used to determine whether the concentration of Cr(VI) is over 0.1%; they can only be used to indicate the amount of CR(VI) per m² of the tested piece. Chapter 5 describes how to interpret the result of a laboratory analysis.
Risk of false positive results
The experiments have yielded no false positive results. However, the method is unsuitable for red-enamel painted pieces that may release colour to the test solution.
Risk of false negative results
False negative results may be obtained if iron parts are exposed which may release iron that reacts with chromium(VI). Make sure all pieces have intact surfaces (except deliberately abraded surfaces).
It may be difficult to abrade the surface to remove enamel, and for enamel-painted pieces there is a great risk of not exposing the relatively thin layer of chromium(VI) because in most cases the chromium layer will be much thinner than the enamel layer. Laboratories performing tests according to the standard will have similar problems abrading the surface, however.
The method is destructive
After testing, the piece will not show that it has been tested, but because part of the passivation layer is removed, the piece will subsequently be more exposed to corrosion.
It should be noted that a test kit (e.g. the Spectroquant Chromate cell test) can be used for screening boiling tests, but this may require a new colour scale, because in terms of chemicals the test kit is not completely identical with the spot test liquid in the IEC test. IEC 62321, 2008 also describes a test liquid more durable than the spot test liquid based on a solution of diphenylcarbazide in acetone.
4.2.4 Decision tree
The decisions to be taken in connection with the screening test are illustrated in Figure 4.
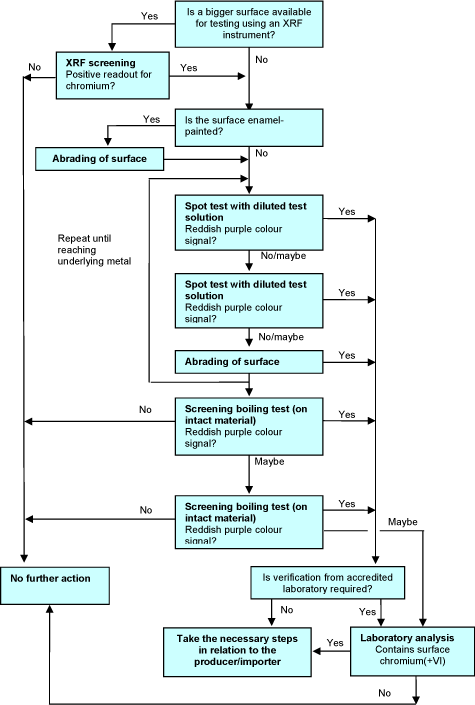
Figure 4 Decision tree for chromium(VI) screening test.
5 Laboratory methods to determine Cr(VI), PBDEs and PBBs
- 5.1 Determination of PBDEs and PBBs in polymers
- 5.2 Determination of hexavalent chromium in surfaces
- 5.3 Colometric determination of chromium(VI) in materials
The International Electrotechnical Commission published an international standard in January 2009, including procedures for the determination of RoHS substances in electrotechnical products, IEC 62321³.
The standard will be available from IEC or Danish Standards.
Neither the RoHS Directive nor the Danish Executive Order prescribes that analyses should be performed in accordance with a particular standard, but in future, IEC 62321 will obviously be used internationally to test electrical and electronic products for RoHS compliance.
A detailed description of how to perform the analyses can be found in IEC 62321, and the description below only covers the analysis principles.
5.1 Determination of PBDEs and PBBs in polymers
IEC 62321 describes one method for determination of PBDEs and PBBs in polymers: gas chromatography combined with mass spectrometry (abbreviated GC/MS).
The first step is to extract the substances from the plastic - in many cases a time-consuming process requiring harsh solvents. The solvent used depends on the type of plastic, which, if unknown, it is necessary to determine by means of infrared spectroscopy or similar methods. Possible solvents include acetone, tetrahydrofuran, toluene, hexane, methylene chloride, chloroform and methanol. According to the standard, an exact determination of PBDEs or PBBs cannot be expected for polymer types that are not easily soluble.
After extraction, inject the sample into a gas chromatograph in order to separate the substance congeners and determining the congener concentration using a mass spectroscope connected with the gas chromatograph.
The results will typically be reported as the sum of the different groups of congeners as follows:
| decaBDE | : | mg/kg |
| ∑ nonaBDE | : | mg/kg |
| ∑ octaBDE | : | mg/kg |
| ∑ heptaBDE | : | mg/kg |
| etc… | ||
| ∑ PBDE | : | mg/kg |
The total concentration of PBDEs is calculated by adding the concentration of all PBDE congeners (a similar procedure is used for PBBs). As mentioned in Chapter 2, the technical products will typically contain a mixture of several congeners.
Price
Presumably, the estimated price of a determination in accordance with IEC 62321 of PBDEs + PBBs using GC/MS in a plastic material will be approx. DKK 10,000 (excluding VAT) for the first analysis and DKK 6,000 – 8,000 (excluding VAT) for the subsequent analyses (February 2009). The high price is due to the very demanding plastic extraction process using various solvents. Using solvent extraction under pressure, ASE (accelerated solvent extraction), would presumably reduce the price to DKK 5,000 – 6,000 (excluding VAT).
5.2 Determination of hexavalent chromium in surfaces
IEC 62321 describes two methods for testing the presence of chromium(VI) in chromated surfaces of metallic pieces and one method for quantitative determination of chromium(VI) in materials.
All the methods are based on colorimetric determination of the chromium(VI) content. The methods are based on the reduction of chromium(+VI) to chromium(+III) in a reaction with 1.5 diphenylcarbazide oxidised to 1.5 diphenylcarbazone, which yields a characteristic reddish purple colour with an absorption maximum of approx. 540 nm.
Spot test
A spot test for the presence of chromium(VI) in metallic surfaces is largely identical with the spot test described in section 4.2.2 (see that section).
Boiling test
IEC 62321 describes a boiling test method to determine the presence of chromium(VI) in a surface. The test is largely identical with the spot test described in section 4.2.3, but differs from that test by requiring pieces with a total surface of approx. 50 cm² and using a slightly different extraction method where the piece has to boil for 10 minutes. The method is specifically described as a test to detect the presence of chromium(VI), but it is not a quantitative method to determine the concentration of chromium(VI) in a material.
The result is typically given in mg/L in the test liquid, which can be converted to mg of chromium(VI)/cm² surface, if the surface area of the sample is known. As the chromium layer constitutes only a very small part of the sample, a conversion to the concentration in the total sample will presumably show a chromium(VI) concentration far below 0.1%. Consequently, the result cannot be used directly to determine whether the sample includes a homogeneous material containing more than 0.1% chromium(VI), and the standard does not provide any instructions as to the interpretation of results in relation to the limit value of 0.1%.
If the thickness of the chromium layer is known, a rough estimate of the concentration of chromium(VI) in this layer can be made. There is no easy method to measure the thickness of the layer, and it is therefore necessary to use literature values for the type of chromium passivation in question. For example, the passivation layer for a yellow passivation on zinc (yellow chromated) is typically 0.3 - 0.6 µm. In connection with the screening method development, calculations of the concentration in the passivation layer have been made for different yellow chromated screws, assuming a layer of 0.45 µm and extraction of all the chromium(VI) in the layer. According to the calculations, a concentration of 0.1% in the surface layer would correspond to measuring approx. 0.1 mg/L in the test liquid (see Annex 1 for more details). It could thus be calculated that the yellow chromated screws tested contained 0.1 - 6.6% chromium(VI) in the 0.45 µm thick surface layer. For blue chromated and bright surface layers that are thinner than the yellow ones, 0.1% in the surface layer would correspond to a lower concentration in the test liquid. It is therefore fair to assume that a concentration of >0.1 mg/L in the test liquid means that there is more than 0.1% in a homogeneous material. This is an interpretation, however, and there is no official statement to the effect that the test results can be converted in this way.
Price
The approximate price of a determination of chromium(VI) using a boiling test is as follows (excluding VAT, February 2009):
- Spot test: DKK 1,500 for the first analysis and DKK 800 for subsequent analyses
- Boiling test: DKK 1,700 for the first analysis and DKK 1,000 for subsequent analyses
5.3 Colometric determination of chromium(VI) in materials
IEC 62321 describes one method for determination of chromium(VI) in materials: The method can be used to determine chromium(VI) in plastic and other homogeneous materials. The method cannot be used to determine chromium(VI) in thin coatings for which the test methods described above should be applied.
As mentioned, the method is based on colorimetric determination of the chromium(VI) content. The method is based on the reduction of chromium(+VI) to chromium(+III) in a reaction with 1.5 diphenylcarbazide oxidised to 1.5 diphenylcarbazone, which yields a characteristic reddish purple colour with an absorption maximum of approx. 540 nm.
The first step is an extraction where about 5 g of the material is placed in an extraction container and chromium(VI) is extracted at 90-95°C with an alkaline solution of sodium hydroxide (NaOH) and sodium carbonate (Na2CO3). The chromium(VI) content in the filtered sample is determined by adding 1.5 diphenylcarbazide and measuring the absorbance at 540 nm using a spectrophotometer. The result is typically given in mg/kg of chromium(VI) and can be used directly to determine whether the material contains < 0.1% chromium(VI).
Presumably, the estimated price of a determination of chromium(VI) in a plastic is approx. DKK 3,200 for the first analysis and DKK 2,500 for the subsequent analyses (excluding VAT, February 2009).
[3] Electrotechnical products - Determination of levels of six regulated substances (lead, mercury, cadmium, hexavalent chromium, polybrominated biphenyls, polybrominated diphenyl ethers)
6 Further information
Relevant information on the RoHS Directive and the RoHS substances can be found in RoHSGuiden.dk (in Danish only) at http://www.rohsguiden.dk (the page will not be updated).
For further information, see the Confederation of Danish Industry's (DI's) guide to the RoHS Directive (in Danish only) at http://www.di.dk/Virksomhed/Miljoe/Miljoe/Producentansvar/Guide+til+RoHS-direktivet/.
For a review of the applications of all RoHS substances in electrical and electronic products, see the report "RoHS substances (Hg, Pb,Cr(VI), Cd, PBB and PBDE) in electrical and electronic equipment in Belgium" at http://www.rohsguiden.dk/imagemanager/imgmanager/uploads/files/RoHS-report-november.pdf.
For updated information on legislation, studies and initiatives in relation to the EU RoHS Directive and the sister directive on waste electrical and electronic equipment (WEEE), see the EU Commission's website at http://ec.europa.eu/environment/waste/weee/index_en.htm.
Version 1.0 May 2009, © Danish Environmental Protection Agency