Waterbased cleaning of mixing vessels
7. Experience with Implementation and Operation of Water-based Cleaning Technology
7.1 General remarks
Because many manufacturers of solvent-borne paints and coatings will, as a rule, have a wide range of products, the technology selected for cleaning mixing vessels and equipment as well as the rinsing solutions used must be suitable for a broad spectrum of products and feedstocks.
Differentiation of cleaning prcesses
For conceptual design of a cleaning plant, generally a range of basic aspects must be considered.
So, for quality reasons it may be necessary to employ different processes for cleaning colored and clear pigmented painting systems. For water-borne coating materials, as a rule the same washing medium may be used, but at most supported by admixing (NMP, n-methyl-pyrrolidone), which forms an azeotrope with water.
For cleaning of isocyanate containing coatings, these processes should be segregated from other systems for known reasons, such as their reaction with water.
As a rule, water-borne paints can only be removed with water or alkaline media while they are still in a wet condition.
Process segregation is to be understood as separation of both stocked materials and the complete piping system in the shop, right up to different nozzle installations. Only this will ensure complete segregation.
Storage
Past experience of operation with alkaline or pure water-based systems has shown that in certain cases unwanted reactions (strong generation of heat) in the rinsing tanks may occur. Frequently, the resulting sediments must be disposed of at great expense.
By deploying an appropriately designed filter system and, as far as needed, additionally equipping the tank farms with agitators, this can be counteracted.
To save investment costs in this area, centralized storage of washing and rinsing media would be desirable. This is widely accepted today as the state of the art.
Logistics
When selecting the washing medium that is optimal for the operator, the existing production logistics must also be considered.
For water-borne paints and air-drying coating systems in particular, the period between filling/emptying and cleaning is decisive. For example, water-borne paints, while still wet, can be relatively easily cleaned with water. But as soon as they become tacky or even dried out completely, they can only be removed with appropriately effective media.
It is best to clean production equipment, such as mixing vessels, immediately after they have been emptied, or at least as long as the paint residues are still wet.
Cleaning of solvent-borne paints while they are still wet using high-pressure water-based jets is problematic, as it is not possible to completely remove the solvent film from the vessel. Cleaning with the paint residues when they are already tacky is therefore usually more satisfactory.
Staff qualifications
Which cleaning medium is optimum in a specific application depends not only on technical and economic considerations, but also on acceptance by the shop floor personnel. A high degree of acceptance will also generally be combined with satisfactory operation as well as high quality servicing and maintenance.
Experience from past practice shows that staff acceptance of an installation for water-based cleaning is greater if personnel receive intensive training, repeated at regular intervals, in handling the equipment, or if they are already familiar with the technology. This applies particularly for companies previously applying solvent-based cleaning processes, and now wish to switch to a water-based system. This is because handling of such systems and coping with the associated risks differ greatly from solvent-based systems.
Regulatory conditions, regulations etc.
When switching from solvent- to water-based cleaning systems in particular, statutory requirements and regulations, for example pertaining to pollution control, maximum allow concentrations (MAC) at places of work, protection of land and water resources etc., must be included in the considerations.
General considerations for cleaning installations
When selecting the optimum cleaning medium, generally operational trade-offs must be accepted. When choosing the cleaning technology, this should not be the case. The cleaning technology must be matched to the specific technical and economic constraints and conditions prevailing at the site and for the production facilities.
7.2 Cleaning Agents
Water
Due to its low cost and easy handling, water without additives is always a very economical cleaning medium. It is primarily used for cleaning emulsion paints in washing installations equipped with brushes or with pressurized jets.
Alkaline solution
Alkaline solution is a mixture of water and, for example, caustic soda at a concentration of 5% to 15%. For maximum effectiveness, this has to be brought up to a temperature of 60o to 80oC . Hot water with a low content of carbonate and magnesium is used for rinsing.
Up to about 20 years ago, caustic soda was used primarily for cleaning. Such media are suitable for cleaning off most water- and solvent-borne paints for protection of structures, wood, motor vehicles, general industrial applications, household needs and most printing inks. Heightened risks to persons, loss of quality due to solution residues, increased operating and capital costs as well as stricter legislation governing their disposal meant that these washing media have little chance of survival over the long term.
Special cleaning agents
These cleaning agents are usually a mixture of potassium hydroxide, tensides and high-boiling point solvents. They are highly effective, and may be used in nearly all sectors. They find application wherever all other media have produced unsatisfactory cleaning results. Basically, these are very efficient cleaning agents, suitable for universal application. Originally, these products were created for stripping paint from wood and metal surfaces etc. Usually, they are supplied in the form of gels. It must be borne in mind that these special cleaning agents cost three to four times as much as NaOH solution, and their disposal is more problematic.
Flammable solvents
Where flammable solvents are used, they consist usually of a regenerate (blend) of several solvent components that are normally contained in the paint and coating products. To reduce costs in general, a non-volatile base solvent is employed, which is then enriched with efficient solvents. Preferably the operator optimizes the composition.
The following must be borne in mind when selecting the composition:
| low toxicity | |
| inflammability | |
| non-volatile | |
| rapid, effective action |
For rinsing, a regenerate of identical composition is used.
Problems arise because of the high vapor pressure of the solvents used for cleaning, they must be explosion-proof and cleaning pressure is limited to 50 bar so that static electricity will not build up. A system for nitrogen purging could be installed, but this is not necessary if equipment and installations are designed according to the regulations.
Non-flammable solvents
Non-flammable solvents exhibit a high flash point (according to the manufacturer's information). Among the solvents falling under this classification are:
| DBE = di-basic ester (dimethyl esters of adipic acid, glutaric acid and succinic acid). |
| NMP = n-methyl-pyrrolidone |
For rinsing, a non-hazardous solvent of identical structure is used.
A disadvantage of both these chemicals is that because of their low vapor pressures these solvents do not dry as quickly as the previous mixtures of volatile and quickly evaporating solvents. A film remains on the vessel surface and needs to be washed away with warm water after the solvent cleaning process. A wastewater treatment plant for this rinsing water is required.
Solvent-based cleaning equipment therefore needs a certain amount of redesign of the cleaning process.
A further decisive factor is the high costs for capital investment and for residues disposal.
Washing medium containing active substances
To be understood by this term products by ALKAREN etc. ALKAREN were used as cleaning agent in earlier tests, see Appendix B.
For rinsing, hot water with a low content of carbonate and magnesium is used.
7.3 Cleaning technology
The cleaning medium is allowed to soak into the residue on the equipment to be washed - a chemico-physical process - and this is then dislodged by the mechanical action of the jet and/or rotating brushes. The washing process consists of several steps that are combined and varied depending on plant type, equipment design and cleaning task. Common to all washing processes is the strict separation of cleaning medium from rinsing water, so that there will be no dilution or concentration of the media and they can continue in use for longer without the need for replacement. Optimized technology will result in proper functioning of the equipment.
Appendix A.1 shows a schematic of water-based and alternative cleaning processes. Examples of implemented cleaning plants are shown in Appendix A.2.
The usual washing process is as follows:
| main wash with alkaline solution (NaOH 15% or KOH; stripper) at a temperature of 60°C to 80°C and pressure up to 80 bar | |
| rinsing step of approx. 10 s with water (with recirculated rinsing water) to remove most of the alkaline solution and to cool down the vessel | |
| final rinsing step with fresh water to remove all remaining alkaline solution from the vessel | |
| venting of steam from the closed washing chamber to atmosphere (drying step) |
The cleaning time is 5 to 15 minutes, dependent on chemical and physical characteristics of the paints or coatings.
The washing water has to be directed to a wastewater treatment plant (e.g. chemico-physical treatment and/or thermal treatment). This wastewater treatment plant has to be attended by skilled personnel. After about four months, alkaline solution becomes spent, and has to be disposed of. Recovery of spent alkaline solution would be very expensive, and is not economic.
The washing action could be provided by a high pressure rotating nozzle or by a rotary brush. Cleaning with brushes is usually done at a lower pressure of up to 10 bar. The advantage of brushes is the mechanical cleaning action and dislodging of paints adhering to the vessel wall. Their disadvantage is that they are subject to wear and have to be changed after approximately 100 –150 cleaning operations.
A further factor to consider is the material of the vessel and the valves. Posing a problem is mild steel, since the cleaning process will also scrape away the surface protection layer, so that it will be exposed to moisture in the ambient air and will corrode. Non-ferrous metals are problematic because they are not resistant to hot alkaline solutions.
This means that if the operator switches to or adopts water-based cleaning, the mild steel vessels will have to be replaced completely with stainless steel ones, giving rise to high costs.
7.3.2 Action of pressure jet and mechanical cleaning using brushes
The pressure jet technique is based on a combination of hydrodynamic energy, admixed chemicals that decompose and dissolve paint and coating compounds, the time factor as well as effective guidance of the pressure jet itself.
Pressure, flow rate and temperature of the washing medium likewise play important parts.
The harder the residues have become, the better is the action of the liquid jet. Example: cleaning of residues that have become rubbery in their consistency is generally only possible with difficulty, or with the application of a very high hydrodynamic energy.
Brush cleaning technique
The brush technique is based on a combination of mechanical surface treatment, admixed chemicals that decompose and dissolve paint and coating compounds, the time factor as well as contact of brushes with the entire surface to be cleaned.
The washing medium itself is generally of lesser importance, as the actual removal action is achieved by mechanical means. Basically it has two functions: supporting destruction and softening of the residue compounds and transportation away of the residue-medium mixture.
Alternative technologies
Alternative technologies, like sandblasting, ultrasonic cleaning, plasma cleaning, freezing and application of dry ice pellets are tried out from time to time, but up to now have not met with any notable acceptance.
7.4 Personnel protection and safety precautions
The risk of handling solvents is well known. Much effort has to be done to protect the operators against inhalation (neuro toxic effects) and physical contact (absorption through skin). Risks due to inflammability and explosions have to be eliminated.
The risk when handling cleaning processes with water-based/alternative cleaning agents, depending on the aggressiveness, can also be high, out of another character.
Dangers arise less during the actual washing process in the closed system or covered vessels and containers and more when filling and emptying mobile tanks and due to the risk of coming into contact with cleaning media during maintenance work.
Appropriate protection equipment and training of the operating staff are necessary, both for solvents and water-based/alternative cleaning agents.
7.5 Environmental impact
7.5.1 Emissions to surroundings and within working areas
Emissions of fumes to the surroundings or within working areas are appreciably less than when working with solvent cleaning equipment. Instead, pollutant emissions are shifted to the wastewater as shown in Figure 7.1.
During operation of the equipment as intended, there shall be no possibility of emissions to the working areas.
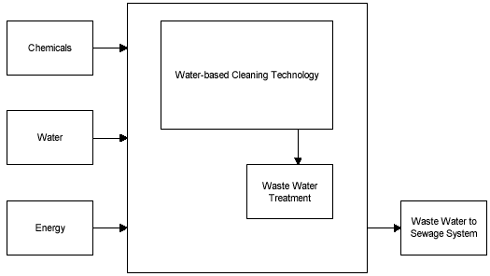
Figure 7.1
Emissions to surroundings.
7.5.2 Wastewater treatment
The rinsing water with its content of cleaning medium as well as the spent cleaning medium, must be directed to a wastewater treatment facility before discharge to the sewer system, or disposed of in some alternative way.
Possibilities are:
| disposal of wastewaters by a specialist company | |
| directing the water to an on-site wastewater treatment plant | |
| distillation of wastewater, recycling the distillate as rinsing water and disposal of residues through a specialist company | |
| chemico-physical wastewater treatment (precipitation/flocculation) |
The wastewaters contain primarily paint and coating constituents, such as resins, pigments, small amounts of solvent etc. and residues of cleaning agents, like alkalies, tensides, salts and small amounts of solvent.
Characteristic parameters are:
| pH from 9.5 to14 | |
| solids content up to 3% | |
| COD value up to 40 g/l |
For treatment, chemico-physical processes or distillation are suitable. However, thermal wastewater treatment is only economic if primary energy is already available.
Manufacturers of water-based cleaning systems normally also supply the required wastewater treatment plants in collaboration with qualified suppliers.
In Appendix A.3 a standard solution of wastewater treatment is shown. The plant consists of buffer tank, reactor for neutralization and coagulation, belt filter for dewatering.
The treated water can normally be let to the municipal sewage system. Sludge from the belt filter has to be disposed of as chemical water.
Normal operation time for treatment of 900 l will be approx. 1 hour.
7.5.3 Residues
The spent alkaline solution has to be disposed of after around four months’ use. Recovery of spent alkaline solution is very expensive and is not economic.