Effects of reduced pesticide use on flora and fauna in agricultural fields
2 Wild flora
(Jensen, A.-M.M. & Johnsen, I.)
2.1 Pilot studies
The purpose of the initial studies was to select, evaluate and adjust methods for the analyses of weed vegetation in a large-scale field study with different levels of pesticide application. The pilot study was used to find the right methods and a dimensioning of the main study that was adequate to make the collection of a sufficiently large amount of data possible despite large variations in weed populations and pesticides.
During 1996 – the pilot year – investigations were performed at one farm: Gjorslev. Two fields were selected for the pilot studies, one grown with spring barley and one with sugar beets. Time of sprayings and normal dosage of pesticides used on the fields appear in Appendix A.1.
2.1.1 Methods (pilot study)
Three different vegetation studies were performed during the pilot year (1996):
- Biomass determination of selected weed species in combination with determination of plant developmental stages (phenology).
- Observations of weed density, cover and number of weed species in permanent sites.
- Preliminary investigations of the seed bank and the seed rain.
Biomass is a well known variable responding on herbicide use (e.g. Kudsk 1989, Salonen 1992b, Olofsdotter et al. 1994). In addition, the plant biomass is positively correlated to the seed production (e.g. Thompson et al. 1991, Hald 1997). Thus a high biomass produces more seeds available for seed eating birds and insects. The development stage is determined from the numbers of leaves, presence of buds, flowers, seeds etc. on a plant. The presence of flowers and seeds are important for the fauna that eats pollen, nectar or seeds. Thus the developmental stage indicate where in the development phase from seedling to seed setting the plant is, not whether the biomass of the individual plant is high or low. Evidently, the developmental stage and the biomass are positively correlated in a population. The cover of the vegetation is positively correlated to the biomass (e.g. Smartt et al. 1974) within a more or less homogeneous vegetation community. However the growth forms of the species influence cover markedly. The density of plants is also assumed proportional with the biomass, provided each plant has the same average biomass despite the density of plants. However this is often not true as several studies have revealed that the average biomass per plant decreases at high plant densities (e.g. Watkinson 1980).
It has been shown that high weed biomass, density and cover support high faunal density (Chiverton & Sotherton 1991, Moreby 1997). The number of plant species in a vegetation community reflects the diversity of the community, and high numbers of plant species often lead to high numbers of animal species.
2.1.1.1 Determination of biomass and phenology
Only weed species with high densities were selected for this study: Viola arvensis in the spring barley field, and Aethusa cynapium, Atriplex patula and Bilderdykia convolvulus in the sugar beet field. For each species, twenty plants from three to five random quadrates in each plot were sampled at four different days during the growing season (May to September). This resulted in a minimum of 60 individual plants for analysis per dosage per collection. The twenty plants were collected in the order of observation within a quadrate. The developmental stage of the collected plants was determined after the BBCH-scale (Hansen et al. 1995) immediately after collection by counting the number of true leaves, branches, buds, flowers and fruits. To measure dry biomass, plants were dried at 80o C for 24 hours. Biomass data were log-transformed before means were calculated (=geometric means) and analyses of variance were performed. Means of biomass were analysed parametricly and medians of development stages were analysed non-parametricly by a Kruskal-Wallis test.
2.1.1.2 Observations of density, cover and number of weed species
In every plot, 5 permanent subplots of 25 m x 25 m were chosen at random. The distance to other plots, hedges, habitat islands etc. was always larger than 12 m to avoid impact from farming operations on the field headland, which may differ considerably from those practised on the experimental field (Fielder 1987). Vegetation in headlands and field margins often differ from the inner part of a field with respect to plant density and species composition (Marshall 1989, Wilson & Aebischer 1995).
In each of these 625 m2 subplots a number of smaller sampling sites were selected for non-destructive vegetation observations. Hence, four random sampling sites were selected in the spring barley fields and 10 in the sugar beet field. Each sampling site measured 0.6 m x 0.4 m = 0.24 m2 and was marked, so it could be visited several times during the season. At each visit, the weeds (seedlings, vegetative or generative plants) were identified to species according to Haas & Laursen (1994), Hanf (1990) or Hansen (1981). In addition, the cover of each species was estimated on a scale from 0 % to 100 % cover of the soil surface. The same person performed the identification of plants and the subjective estimates of cover during the whole season. Values of density, cover and number of weed species were summed up to the level of subplots. Weed density was log-transformed and cover was square root transformed to improve approximation to normal distribution before data were analysed for effect of dosage. Data from each visit were analysed separately.
2.1.1.3 Seed bank and seed rain
The viable seed bank population was studied in both fields in August by sampling five soil cores at six random places per plot, each core was 2.0 cm in diameter and 10 cm in depth. In September, samples of the seed rain were obtained from another six random places per plot. Each seed rain sample was extracted from the soil surface of five 0.1 m2 squares using a house vacuum cleaner connected to a transformer placed in the boot of a van. The number of seeds in the seed bank and seed rain samples was estimated and identified to species by allowing the viable seeds to germinate. After approximately a month the seed samples were spread as a 5 mm layer upon a 3 cm thick sterile substrate of vermiculite and permaculite and placed in a unheated greenhouse with presumably very little influx of weed seeds. Germinating seedlings were identified to species, counted and removed. The germination trays were observed for 16 months and watered when necessary. Trays without seed sample addition were left in the greenhouse for control of sterility and any possible seed influx. This germination method has been used with satisfactory results during most of the last century to reflect the amount of viable seeds in the soil seed bank of agricultural land (e.g. Jensen 1969, Roberts 1981).
2.1.2 Results (pilot study)
2.1.2.1 Determination of biomass and phenology
Biomass of weed species in sugar beets
From the fourth collection in September (Fig. 2.1), the mean dry biomass of Atriplex patula was significantly higher in the plot sprayed with quarter dosage than in the plots sprayed with half or normal dosage (Tukey-Kramer tests, p=0.013 and p<0.0001, respectively). No significant effect of dosage was seen in biomass of Aethusa cynapium or Bilderdykia convolvulus at this collection.
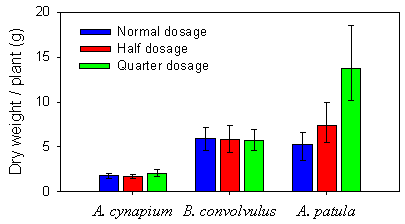
Fig. 2.1.
Mean dry biomass (g) per plant for Aethusa cynapium, Bilderdykia
convolvulus and Atriplex patula collected mid-September (fourth
collection) in a sugar beet field treated with three different dosages of pesticides.
Error bars represent the 95 % confidence limits.
The higher biomass of Atriplex patula at quarter and half dosages than at normal dosage was significant from second collection already.
Only at second collection in late June, plant dry weight of Bilderdykia convolvulus showed an effect of dosage. Plants at quarter dosage had a significantly higher biomass than at half dosage (Tukey-Kramer test, p=0.005) and the plants at half dosage had a significantly higher biomass than plants at normal dosage (Tukey-Kramer test, p=0.001).
At third collection in late July a significant effect of pesticide dosage was observed for dry biomass of Aethusa cynapium, mean dry biomass was higher at quarter dosage than at normal dosage (Tukey-Kramer test, p=0.002).
Biomass of Viola arvensis in spring barley
Very few Viola arvensis plants survived spraying with normal herbicide dosage in spring barley, not enough for statistical analyses, thus no observations on biomass and developmental stages were performed in the spring barley plot receiving normal dosage. Immediately before harvest of spring barley in August, significantly smaller V. arvensis plants (mean 13 mg) were found in the plot receiving half dosage in comparison with plants in the plot receiving quarter dosage (mean 23 mg) (t-test, p=0.002). No difference, however, was observed at any other time of collection.
Phenology of Aethusa cynapium
Five weeks after spraying, A. cynapium showed a significant difference in developmental stages at different herbicide dosages in the sugar beet field (Kruskal-Wallis test, p<0.001). Plants treated with quarter dosage were significantly more developed than plants treated with normal dosage (Fig. 2.2). This pattern was found at the first three collection days, while at last collection the differences in developmental stages among dosages were no longer significant (Kruskal-Wallis test, p=0.08).
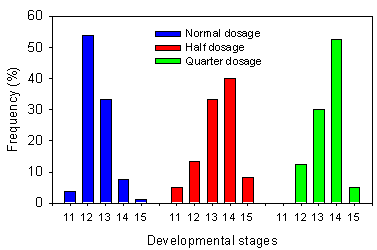
Fig. 2.2.
Distribution of developmental stages for Aethusa cynapium in
the sugar beet field mid-June. A higher figure on the x-axis corresponds to a higher plant
developmental stage.
Phenology of Atriplex patula
The developmental stages for A. patula showed significant differences only at the second seasonal collection, plants sprayed with half or quarter dosage were at a higher developmental stage than plants sprayed with normal dosage (Kruskal-Wallis test, p<0.0001).
Phenology of Bilderdykia convolvulus
The developmental stages for B. convolvulus showed no differences between the three levels of pesticides at any of the seasonal collecting times.
Phenology of Viola arvensis
The developmental stages of V. arvensis were significantly higher at second and fourth collecting day for the spring barley plot receiving quarter dosage compared with the plot receiving half dosage (Wilcoxon tests, (p=0.17), p=0.02, (p=0.26) and p<0.001, respectively).
2.1.2.2 Observations of density, cover and number of weed species
Density and cover of weeds in sugar beets
Total weed density at quarter dosage rose from week 30 to week 40 (Fig. 2.3A) because of germinating seedlings originating from seed shedding plants in the field (especially Poa annua). The confidence limits for weed density at quarter dosage were huge mainly because one of the subplots had a three times higher density than the other four. The total cover of weed increased from under 0.5 % in the beginning of the season to 4 % on average at the end of July (week 30) (Fig. 2.3B). The cover decreased to 3 % on average in week 40 because some species defoliated. During the season, changes in total cover were greater than changes in total density.
Fig. 2.3.
Development in total weed density (A) and weed cover (B) during the growing
season in a sugar beet field sprayed in week 19 and 20 with normal or reduced dosages of
herbicides. Every dot is a geometric mean of five values. Error bars represent the 95 %
confidence limits
Dosage had a significant effect on total density in week 23, 26 and 30 (anovas) giving higher plant density at quarter dosage than at half dosage. The cover was only significantly affected by dosage in week 26 (p=0.014) (Fig. 2.3B).
The most numerous species in the beet field was Aethusa cynapium, whereas more than 50 % of the total weed cover was contributed by Bilderdykia convolvulus.
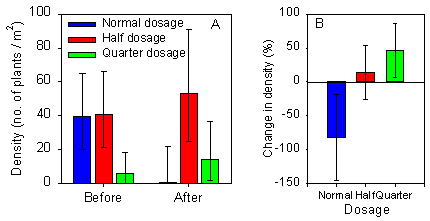
Fig. 2.4.
A) Density of Viola arvensis in spring barley before and
after spraying with normal or reduced dosages of pesticides. B) Change in density of Viola
arvensis after spraying, where density before spraying is set to 100 %. Each
column represents the mean of five values. Error bars represent the 95 % confidence
limits.
Density of Viola arvensis in spring barley
Before spraying, the average density was very low in the quarter dosage plot and much higher and nearly equal in the half and normal dosage plots (Fig. 2.4A).
After spraying with normal dosage the average density of V. arvensis decreased markedly and the average densities in plots sprayed with half and quarter dosage increased (Fig. 2.4B).
Number of weed species in sugar beets
The highest number of species was present in the beginning of the growing season (Fig. 2.5). Number of species was decreasing at the same time as the total density was decreasing. No significant effects of dosages were found (anovas).
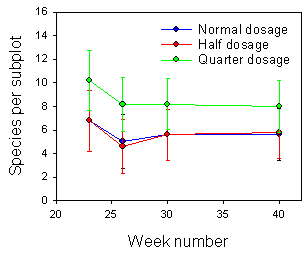
Fig. 2.5.
Development in number of weed species during the growing season in a sugar
beet field sprayed with normal or reduced dosages of pesticides in week 19 and 20. Error
bars represent the 95 % confidence limits.
2.1.2.3 Seed bank and seed rain
In the control trays seedlings of Chamomilla sp., Conyza canadensis, Epilobium sp., Erophila verna, Poa annua, Sonchus sp. and Taraxacum sp. germinated. These seeds may have been in the substrate or more likely must have spread from the surroundings into the greenhouse. They are all wind dispersed or grew near the greenhouse. Three of the species (Chamomilla sp., Poa annua and Taraxacum sp.) were observed in the field vegetation. Therefore, it is not possible to clarify whether the seeds of these species were in the soil samples from the field or whether they have arrived from outside the greenhouse. Fig. 2.6 shows the distribution of germinated seeds from the samples grouped according to the proportion of seeds from species also found in control trays, species from the fields, grains and unidentified seeds, respectively. In total 15,921 seedlings were identified from the seed bank and the seed rain. More than 50 % of the seedlings were of species also found in the control trays. Only 8 % of the seedlings were identified as weed species originating from the field.
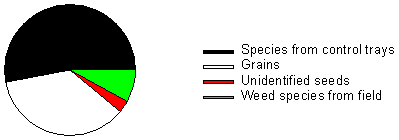
Fig. 2.6.
Total number of germinating seeds in seed rain and seed bank samples
distributed on species from control trays, grains, unidentified seeds and weed seeds from
the field.
2.1.3.1 Determination of biomass and phenology
The results of the determination of biomass provided good information on weed response after spraying with reduced dosages in the fields (which have also been shown by several others (e.g. Landbrugets Rådgivningscenter 1999b)). Five of twelve analyses showed a significant effect, in all cases with a higher biomass at quarter dosage than at normal dosage. The changes in weed developmental stages among dosages (and collection days) seem to reflect well the impact of pesticide application on phenology. Six of sixteen analyses showed a significant effect, which in all cases with a higher developmental stage on average at quarter dosage than at normal dosage.
For measurements of biomass and developmental stages, many plants of the same species at each treatment are needed, because of a very large variation between plants and a small variation between dosages. In addition, the investigation demands a very even distribution of plants of the same species in the three plots in a field. In many fields, this would not be possible to achieve except for the most abundant species. Furthermore, the method is very time-consuming, and would be impossible to perform on a sufficient area within each plot in the large-scale field study. In conclusion, the determinations of biomass and developmental stages were abandoned in the main experiment 1997-1999.
2.1.3.2 Observations of density, cover and number of weed species
Density and cover were measured four times through the growing season. Density was more stable than cover after week 26, where the mortal effect of the herbicide treatments had stopped. Thus, measurements of cover vary much over the growing season, as also found by Hill et al. (1989) for several species. However, greater variation in the measurements of cover than densities results in less significant differences between treatments. In this experiment, significant effects of reduced pesticide dosages were found three times in the analysis of total density and only once in the analysis of cover. Furthermore, cover is a subjective personal estimate and differs from person to person (Kennedy & Addison 1987). Therefore, variation would be greater in the main study, where several persons would evaluate the cover, than in this one-year study, where only one person estimated the cover. If a more exact analyse of cover was performed (e.g. a pinpoint analyse) the time used would at least be trebled. However, the advantage would be that exact estimations of cover might give a better estimate of the competitiveness of the weed species (Silvertown & Dale 1991) compared to density measurements. Densities tell nothing about the size of the plants and, hence, how well they compete with the crop for light and nutrition. Plant biomass might still be a better indicator of the food resource available than density of plants of unknown size, even though the two measurements are positively correlated. However, the density is more stable during the last half of growing season than the biomass (which may follow the same flutuations as cover) and may therefore be more useful in analyses of fauna densities, since the large-scale study only allows one registration during each season. On balance, it was decided to abandon the measurements of cover and retain the measurements of density in the main experiment 1997-1999.
Repeated observations of the vegetation through the growing season are time-consuming and contribute little additional information because data are not independent (counting the same plants several times). Figs. 2.3 and 2.5 show that density and number of species do not change after week 26 and until new seedlings germinate. Thus, one registration after June gives sufficient data to determine differences in vegetation density and richness, in relation to the use of pesticides. This agrees with Hald & Lund (1994), who measured weed densities in unsprayed fields at two periods during springtime and did not find any great difference in densities between the periods.
The development in density of Viola arvensis in spring barley (Fig. 2.4) illustrates that it is very important to know the differences between plots before spraying; else false conclusions may easily be drawn. This knowledge can be achieved by observing the vegetation before spraying and use that observation as a covariate in the analyses of the vegetation after sprayings. In the main study, the density and number of species were registered before spraying (in April-May) and once after spraying (in July-August).
No significant effect of dosages on species number was found in the sugar beet field. This might be due to the small area of investigation in the sugar beet subplots. The weed density in the sugar beet field was much lower than the density in the spring barley field, suggesting that the occurrence of different species would also be more scattered in the sugar beet field. If the recorded number of species is low compared to the maximal number of species found in that habitat, the variation between samples will be greater than if the number was close to the maximal number of species possible. Consequently, the sampling sites in sugar beet fields were doubled in size and a higher number of samplings sites were investigated in the main experiment.
2.1.3.3 Seed bank and seed rain
The germination method for seed bank samples proved unsatisfactory, because of the very high contamination of the samples with many arriving seeds from the environment germinating in the trays. Contamination under germination experiments in greenhouses is usual; Jensen (1969) found a contamination of 4 % of the total number of germinating seeds. Nevertheless, in the present experiment the amount of incoming seeds was unacceptablely high. Thus, another method for measuring the seed rain had to be used in the main experiment.
2.2 Vegetation studies
During the main phase of the study (1997-1999), two different kinds of studies were performed, a vegetation study (section 2.2) and a seed rain study (section 2.3).
The objectives of the vegetation study were to detect any effects of reduced pesticide dosages on the wild flora, measured by several vegetation parameters such as density, species richness (=weed diversity), species composition and ability to flower. Data were obtained from 15 experimental fields: five of each crop (spring barley, winter wheat and sugar beets) over a period of three years. The aim was not to study the effectiveness of the herbicides to control (kill) the weed flora and correlate this effectiveness with the individual treatments of individual herbicides, such studies have been performed in many small field trials (e.g. Landbrugets Rådgivningscenter 1999b). Rather, it was aimed to measure changes in the weed flora parameters that might have an impact on the existence of the fauna on the fields.
The objective of the seed rain study was to detect effects of reduced pesticide dosages on the number of species and density of seeds in the seed rain in early autumn. Data was obtained from 4 winter wheat and 5 spring barley stubble fields in 1998 and 4 winter wheat and 4 spring barley stubble fields in 1999.
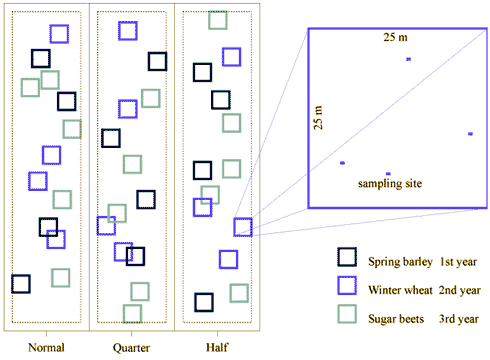
Fig. 2.7.
One example of locations of plots (Normal, Quarter and Half), subplots and
sampling sites (in magnified subplot) in a field grown in a three years rotation with
spring barley, winter wheat and sugar beets. Punctuated lines indicate the 12 meter zone
to hedges etc.
2.2.1 Methods
2.2.1.1 Sampling design
Field design is illustrated in Fig. 2.7. In each plot, subplots of 25 m x 25 m were chosen at random with at least 12 m to other plots, hedges and habitat islands such as small game plantations, ponds etc. Non sowed tramlines were avoided. 5 subplots were chosen in each sugar beet plot and 4 subplots in each cereal plot.
Location of the subplots within a plot varied from year to year. Within the sugar beet subplots ten sampling sites of 1.0 m x 0.5 m were placed at random. Within the cereal subplots, four sampling sites of 0.6 m x 0.4 m were placed at random. These sampling sites were used for the vegetation studies. The total area used for vegetation observations per subplot was thus in the sugar beet fields 5.0 m2 (10 x 0.5 m2), and in the cereal fields 0.96 m2 (4 x 0.24 m2). The different area of subplots and their numbers were chosen on background of results from the pilot study (section 2.1.3.2).
2.2.1.2 Vegetation measures
The vegetation study included identification of the weed species present as well as counting the number of individual plants per species within the sampling sites. Data from the individual sampling sites within the same subplot were summed up giving the total number of species present per subplot and the density of plants per species and in total per square meter. The reasons for selections of these parameters are given in section 2.1.3.
2.2.1.3 Number and timing of field observations
As a consequence of results found in the pilot study (section 2.1.3.2), each sampling site was examined two times during the growing season, once immediately before sprayings with herbicides in spring (April-May) and once approximately 3 months later (July-August).
Eight of the winter wheat fields were sprayed with herbicides both in autumn and in spring, four only in autumn and three only in spring. For a better comparison of effects of spring spraying with sugar beet and spring barley fields, only data from the eleven winter wheat fields sprayed in spring entered the analyses and figures. Observations of arthropods and birds were not carried out in winter wheat fields before spring, so only effects of spring sprayings were analysed in the vegetation. The weed observations before spring sprayings were used as a covariate in the analyses of weed variables after spraying in that way the effects of soil cultivation and autumn sprayings on the weed populations were included in the covariate. The exclusion of winter wheat fields only sprayed in autumn was due to an expectation of very few and only minor changes in the weed populations between spring and summer caused by dosage differences in the autumn sprayings.
2.2.1.4 Flowering status
After treatment with pesticides in 1998 and 1999, the numbers of flowering plants per species were recorded in all sampling sites. A plant was defined as flowering if it was generative, i.e. if it had developed buds, flowers, fruits or showed any sign of fruit setting (e.g. empty sepals in Lamiaceae). The aim was to detect whether pesticide dosages had sublethal effects on weed plants by reducing fitness, measured as ability to flower. In addition flowering plants provide pollen and nectar for insects and later in the season seeds for both insects and birds, whereas vegetative plants provide less energy-dense food. The proportion of flowering plants was calculated as the number of flowering individuals of all species divided by the total sum of plants across all species in a plot. The proportion of flowering species was calculated as the number of species, where at least one plant did flower, divided by the total number of species in a plot.
2.2.1.5 Habitats in sugar beet fields
At reduced dosages in the sugar beet fields two different microhabitats arose for the weeds: within the row, where the weeds had to compete with the beets and tolerate/escape the herbicides to survive; and between the rows, where competition from the sugar beets was less strong but the weeds had to survive hoeing. At normal dosage only Gjorslev performed hoeing regularly between the beet rows (Appendix B). In 1998 and 1999, it was noted whether the weeds occurred within the sugar beet rows (closer than 12.5 cm to a row) or between them (more than 12.5 cm apart from a row).
2.2.1.6 Identification and counting of plants
Seedlings were identified using Hanf (1990) or Haas & Laursen (1994), the vegetative grasses using Grøntved & Sørensen (1941) and the flowering plants using Hansen (1981). Nomenclature follows Tutin et al. (1964-1980). All plant species except the grown crop were counted inclusive volunteer plants of previous crops (e.g. oil seed rape plants in a sugar beet field and winter wheat plants in a spring barley field). Determination of densities of perennial plants that either germinates from roots or from rhizomes (e.g. Cirsium arvense and Elymus repens) was based on shoots more than 2 cm apart. Shoots closer than 2 cm were counted as one individual plant.
2.2.1.7 Definition of common, rare, scarce, non-target and target species
Species were divided in different groups: Common species are common or very common all over DK according to Hansen (1981) in contrast to rare species, which is species mentioned as seldom or rare at least in some parts of Denmark. The rare species in this study are not rare in the Southeastern part of Denmark, but occur very seldomly if at all in the Western and Northern parts of Denmark (Mikkelsen 1989). Many of the investigated species appeared only in a few plots, although their overall abundance in Denmark was common. Species found in less than six of the 123 investigated plots were classified as scarce species. Non-target species in contrast to target species are species that do not reduce the crop yield neither by competition with the crop for light, water or nutrients nor by impeding the harvesting, thus non-target species are not unwanted from the farmers point of view. It is evident that the competition from a weed species against the crop plants depends on the density of the weed species and the competitiveness of the crop. Cereals are strong competitors and row-crops as sugar beet are weak competitors. In sugar beets nearly all weed species would be target species, whereas in cereals weed species can be divided in strong or weak competitors. Species with a competitiveness from medium to very strong in winter wheat (Christensen & Rasmussen 1998) were defined as target species. In addition the top ten species with the highest weed equivalents in either autumn sown or spring sown crops were defined as target species (Jensen 1996). All other species were in this report defined as non-target species.
2.2.1.8 Statistical analyses
Response variables
The basic experimental unit in the analyses of all variables was the plot (n=123, the four wheat fields without herbicide sprayings in spring being excluded). Values from subplots within a plot were summed or averaged to one value for each plot each year. Statistical analyses were performed on the following response variables:
- Mean density per plot for all weed plants
- Development of mean total density per plot during the study years
- Mean density per plot for the most common species in cereals, separately
- Density of some rare species, separately
- Total number of species per plot
- Development of species number per plot during the study years
- Abundance of rare species
- Abundance of scarce species
- Proportion of flowering plants per plot
- Proportion of flowering species per plot
In the rest of this section, variable number refers to the above response variables.
Fields, where a species was not present in any of the plots after spraying was excluded in the analyses of density for that particular species (variable 3 listed above), because lack of the species was regarded as non-informative with respect to dosage dependency. A plot was omitted from the data analyses of proportion of flowering species (variable 10) if the number of species found in the plot was less than six, due to high stochastic variation in proportions based on small numbers.
Tests
The null hypothesis was that the means of the response variables were not significantly influenced by pesticide dosages (variables 1, 3, 4, 5, 7, 8, 9 and 10) or year (variables 2 and 6). For the variables 1-6 and 9-10, the hypothesis about differences between dosages or years was tested by means of analysis of variance, taking into account the effect of differences between explanatory factors such as crop, farms, fields, years and weed status before spraying (Table 2.1).
The variables were transformed in order to improve the approximation to a normal distribution and make the variance independent of the mean. The data transformations were accepted after running the model, if the plot of residuals against predicted values did not show any apparent trends.
Effects may have accumulated during the study period because the relative dosages applied to a certain plot were the same in all three years. This was taken into account in the analyses of variable 1-6 by using density or species richness before spraying as a covariate. The total density of plants might influence the number of species found, therefore the density of weed plants was used as a covariate in analyses of variable 5 and 6 and the proportion of flowering plants was used as a covariate in analyse of variable 10.
Notice that dosage was treated as a class variable, so no assumptions were made about the effects of half dosage falling in between those of quarter and normal dosages.
The explanatory factors (main factors and interactions) used in the full models are shown in Table 2.1.
Model reduction was performed using an iterative procedure to remove the variables with p > 0.10 until the model consisted only of variables with p £ 0.10. If a significant effect of dosage was revealed, the differences among dosages were tested by a Tukey-Kramer adjustment for multiple comparisons of least-squares means. The analyses were performed using the GLM procedure in SAS (SAS Institute 1999).Because not all winter wheat fields were used in the statistical analyses, the design was unbalanced, and the F-tests had to be modified, this was done using the Random/Test statement in the GLM procedure.
The proportion of variation explained by a certain factor was calculated as the sum of squares for that factor divided with the total sum of squares.
Dosage effects on the abundance of rare and scarce species (variables 7 and 8) were analysed using chi2-tests. Species or groups of species with very low abundance were summed before tested.
Table 2.1.
Explanatory factors included in the variance analyses. An asterisk indicates
that the explanatory factor is included in the full model for that particular response
variable. df = degrees of freedom.
Explanatory factors |
Included in the full model for variable: |
Description |
Max. |
|||
1, 4, 5 |
3 |
2, 6 |
9, 10 |
|||
Density or species number before |
* |
* |
|
|
diff. in density or species number before spraying |
1 |
Density or flowering proportion |
*only variable 5 |
|
*only variable 6 |
*only variable 10 |
diff. in density or in proportion of flowering plants |
1 |
Dosage |
* |
* |
* |
* |
diff. between dosages |
2 |
Crop |
* |
* |
* |
* |
diff. between crops |
2 |
Farm |
* |
* |
* |
* |
diff. between farms |
4 |
Field(Farm) |
* |
* |
* |
* |
diff. between fields within farms |
10 |
Year |
* |
* |
* |
* |
diff. between years |
2 |
Season |
|
|
* |
|
diff. between before and after spraying |
1 |
Dosage´ Crop |
* |
* |
* |
* |
diff. between dosages vary between crops |
4 |
Dosage´ Farm |
* |
* |
* |
* |
diff. between dosages vary between farms |
8 |
Dosage´ Year |
* |
* |
* |
* |
diff. between dosages vary between years |
4 |
Crop´ Farm |
* |
|
* |
|
diff. between crops vary between farms |
8 |
Crop´ Year |
* |
|
* |
|
diff. between crops vary between years |
4 |
Farm´ Year |
* |
|
* |
|
diff. between farms vary between years |
8 |
Dosage´ Season |
|
|
* |
|
diff. between dosages vary between seasons |
2 |
Crop´ Season |
|
|
* |
|
diff. between crops vary between seasons |
2 |
Farm´ Season |
|
|
* |
|
diff. between farms vary between seasons |
4 |
Year´ Season |
|
|
* |
|
diff. between years vary between seasons |
1 |
Dosage´ Crop´ Farm |
* |
|
* |
* |
diff. between dosages vary between crops and farms |
16 |
Dosage´ Crop´ Year |
* |
|
* |
|
diff. between dosages vary between crops and years |
8 |
Dosage´ Farm´ Year |
* |
|
* |
|
diff. between dosages vary between farms and years |
16 |
Crop´ Farm´ Year |
|
|
* |
|
diff. between crops vary between farms and years |
16 |
Dosage´ Season´ Crop |
|
|
* |
|
diff. between dosages vary between crops and seasons |
4 |
Dosage´ Season´ Farm |
|
|
* |
|
diff. between dosages vary between farms and seasons |
8 |
Dosage´ Season´ Year |
|
|
* |
|
diff. between dosages vary between years and seasons |
4 |
2.2.2 Results
2.2.2.1 Density
Total weed density
Weed densities after spraying changed markedly with dosage, so the density at normal dosage (mean 30 plants per m2) was significantly lower than at half (mean 48 plants per m2) and quarter dosage (mean 55 plants per m2) (Tukey-Kramer tests, p=0.016 and p=0.008, respectively). No significant difference in densities was seen between half and quarter dosage (p=0.96).
Density differences between crops were highly significant (anova, p=0.007) and explained most of the variation in densities. Total density after spraying was on average 84 plants per m2 in spring barley, significantly higher than in sugar beets (32 plants per m2) and winter wheat (28 plants per m2) (p<0.0001 for both comparisons).
Development of weed density
Densities before spring spraying averaged over three crops (mean 98 plants per m2) were significantly higher than after spraying (mean 43 plants per m2) (anova, pseason<0.003) (Fig. 2.8). The density before spring spraying was significantly lower in winter wheat (mean 63 plants per m2) than in spring barley (mean 141 plants per m2) and sugar beets (mean 110 plants per m2), this difference was mainly due to autumn sprayings, winter mortality and a higher competitiveness in winter wheat than in spring sown crops. No significant dosage difference was found in the weed density before spring spraying, though the density at half dosage was higher than at normal dosage with quarter dosage in between.
There was no effect of year on the densities before spraying, but the densities after spraying increased with year independently of dosage (anova, p<0.0028 for year as a continuous explanation factor and p=0.65 for dosage´ year). Thus, no between year differences in the effect of dosage were found, so no traceable accumulation of effects could be detected over the three years.
Densities of seedlings counted prior to implementation of treatments (1997 before) were not significantly different (anova, p=0.72) on plots designed to receive normal, half and quarter dosage of pesticides. Thus, the experimental basis was not biased before the beginning of the study. No effect of dosage was seen on the weed seedling densities before spring spraying in 1998 and 1999, hence after one or two years with reduced pesticide dosages.
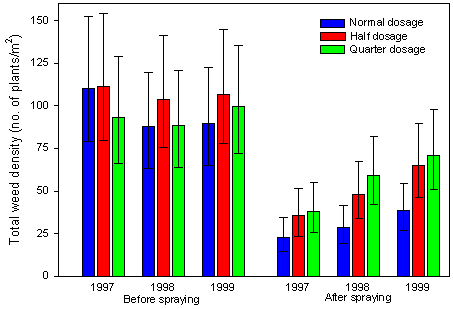
Fig. 2.8.
Development of total weed densities before and after spraying over three
crops during three growing seasons.
Each bar represents the geometric mean of 12 to 15 values of mean density per m2.
Error bars represent the 95 % confidence limits.
Density of common species
Viola arvensis, Poa annua, Veronica sp., Polygonum aviculare and Lamium sp. were the most frequent species/genera, occurring in 75-85 % of the plots after spraying. The ten species/genera that occurred in most plots were also the ten species/genera with the highest overall densities; thus, the frequent species were also the dominant species. Table 2.2 lists the twelve most frequent species/genera, and the effect of dosage on their densities in cereal fields (anovas).
Densities of four species/genera showed a significant effect of dosage. Of those, the density at quarter dosage was higher than at normal dosage in all cases, and in one case, the densities at half dosage were higher than at normal dosage (Table 2.2). The estimated mean density of more than two thirds of the species was higher at reduced dosages than at normal dosage despite the density was only significant different in one third of the species.
Table 2.2.
Species/genera found in more than 50 % of the 123 plots. Effect of dosage on
the density of each species/genus in cereal fields analysed by variance. Each species is
defined as a target (T) or a non-target (NT) species (cf. section 2.2.1.7). Statistical
significant difference from normal dosage is indicated as follows:
+: *: 0.05£ p<0.10, *: 0.01£
p<0.05, **: 0.001£ p<0.01 and ***: p<0.001.
Common species/genera |
Target (T) or non- target (NT) |
Number of plots in the analysis (n) |
Estimated
mean density |
||
normal dosage |
half dosage |
quarter dosage |
|||
Aethusa cynapium |
NT |
54 |
2.4 |
4.2* |
4.9** |
Anagallis arvensis |
NT |
51 |
1.2 |
1.2 |
1.2 |
Bilderdykia convolvulus |
NT |
66 |
1.0 |
1.3 |
1.9* |
Chamomilla sp./Matricaria perforata |
T |
57 |
0.7 |
0.8 |
0.9 |
Chenopodium album |
T |
42 |
0.7 |
1.1 |
1.4 |
Elymus repens |
T |
57 |
1.1 |
0.9 |
1.1 |
Lamium amplexicaule/hybridum/ purpureum |
T |
66 |
1.0 |
1.5 |
1.8* |
Poa annua |
NT |
69 |
4.9 |
5.8 |
6.6 |
Polygonum aviculare |
NT |
66 |
2.7 |
3.4 |
3.4 |
Stellaria media |
T |
66 |
1.3 |
1.8 |
3.1*** |
Veronica agrestis/arvensis/persica |
NT |
66 |
1.9 |
2.5+ |
2.3 |
Viola arvensis/tricoor ssp. tricolor |
NT |
75 |
2.4 |
2.8 |
3.1 |
Density of rare species
Of the rare species (definition in section 2.2.1.7) found in this field study only Euphorbia exigua and Silene noctiflora were found in sufficient numbers for statistical analyses.
Fig. 2.9.
A) Densities of Euphorbia exigua after spraying with
different dosages of pesticides in three subsequent years. Every bar represents the
geometric mean of three plot means from fields at one farm. B) Density of Silene
noctiflora before and after spraying with different dosages of pesticides in 1998
at the spring barley field at one farm. Each bar represents the geometric mean of four
values from different subplots. C) Change of S. noctiflora density after
spraying compared to density before spraying. Error bars represent the 95 % confidence
limits.
Fig. 2.9A shows the densities of E. exigua at different dosages during three years. In 1997 and 1998 there was a significant effect of dosage on density of E. exigua, with a higher density at quarter dosage than at normal dosage. In 1999, no significant dosage effect was found, which might be due to chances. The density of S. noctiflora plants before and after spraying is illustrated on Fig. 2.9B. Because of a huge variation between subplots, no significant effect of dosages was found (anova, p=0.18). However, the figure illustrates that density at normal dosage was lower after spraying than before spraying in comparison to density at half and quarter dosages, which did not decrease (Fig. 2.9C).
2.2.2.2 Species richness
A total of 85 weed species were found within the study plots during the three-year study period (see Appendix C.1). Four of the species were only found before spraying. Of the 81 different species found after spraying 69 of them were broad-leaved species, 11 monocotyledons and 1 pteridophyte. The species grouping by life cycles gave; 4 trees, 18 perennials and 59 annuals of which 30 were purely summer annuals.
Number of species per plot
The dosage had a highly significant effect on the number of species present in a plot (Fig. 2.10). The variation in the covariates (species richness before spraying and total weed density) explained a significant part of the variation in number of species after spraying (Table 2.3). Crop type explained most of the variation in number of species and had a highly significant effect on the number of species present (anova, p<0.0001). The number of species can not be directly compared between the sugar beets and the cereals, because of the different sizes of the investigated areas. There was a much higher number of species in spring barley than in winter wheat.
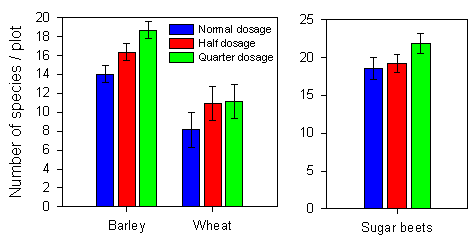
Fig. 2.10.
Number of plant species after spraying with normal, half and quarter dosage
of pesticides in three crops. Cereals and sugar beets are depicted individually since the
areas investigated were of different sizes. Each bar represents the mean of 15 (spring
barley and sugar beets) or 11 (winter wheat) values. The error bars represent the 95 %
confidence limits.
In spring barley, the effect of dosage was significant and explained 17 % of the variation in species number. The number of species at quarter dosage was 14 % higher than at half dosage (p=0.002) and the number of species at half dosage was 16 % higher than at normal dosage (p=0.004). Most of the variation in number of species was explained by the farm´ year interaction, probably reflecting differences between fields.
Dosage had an almost significant effect on species richness in winter wheat (anova, p=0.054), giving 36 % higher species richness at reduced dosages than at normal dosage. Most of the variation in species richness after spraying was explained by variation in total weed density.
Table 2.3.
Schematic summary of the analyses of species richness. Statistical
significance is indicated as follows: +: 0.05£ p<0.10, *:
0.01£ p<0.05, **: 0.001£
p<0.01 and ***: p<0.001. Grey areas indicate explanatory factors not included in the
full model.
|
Species richness |
|||
Factors |
All crops |
Barley |
Wheat |
Sugar beets |
Species richness before |
** |
*** |
** |
|
Weed density |
*** |
* |
*** |
* |
Dosage |
*** |
* |
+ |
|
Crop |
*** |
|
|
|
Farm |
|
|
|
|
Field (Farm) |
* |
|
|
|
Year |
|
|
|
|
Dosage´ Crop |
|
|
|
|
Dosage´ Farm |
|
|
|
|
Dosage´ Year |
|
+ |
|
*** |
Crop´ Farm |
|
|
|
|
Crop´ Year |
|
|
|
|
Farm´ Year |
** |
*** |
|
*** |
Dosage´ Crop´ Farm |
|
|
|
|
Dosage´ Crop´ Year |
|
|
|
|
Dosage´ Farm´ Year |
|
|
|
|
An effect of dosage on species richness in sugar beets existed but varied between years (Table 2.3). No significant dosage effect was detected in 1997 and 1999, but in 1998 a significantly higher species richness was present at quarter dosage than at normal dosage (Tukey-Kramer test, p=0.032).
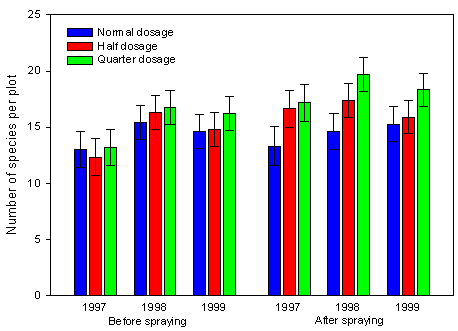
Fig. 2.11.
Development of species richness before and after spraying over three crops
during three growing seasons. Each bar represents the mean of 12 to 15 values. Error bars
represent the 95 % confidence limits.
Development of species richness
No significant effect of year was found on species richness neither before nor after spraying (Fig. 2.11).
The species richness found prior to implementation of treatments (1997 before spraying) was not significantly different on plots intended to receive normal, half and quarter dosage of pesticides, giving the optimal base for the experiment. There was a significant difference in species richness before and after spraying (anova, p=0.01). The species richness after spraying was on average 12 % higher than before spraying. This is mainly due to an artefact caused by a more exact identification of plants after spraying than before spraying. Before spraying, some seedlings could be identified to genera only, whereas most plants could be identified to species after spraying.
2.2.2.3 Species composition and occurrence of rare and scarce species
The dominant weed species were the same in a particular field from year to year, despite the different crops grown on the field, but varied considerably from field to field. Appendix C.1 lists the occurrence of particular species in plots sprayed with normal, half or quarter dosage. Most species, occurring in more than a few plots, did grow at all three dosages, but often with the lowest occurrence at normal dosage. A few species were most common at one or two levels of dosages: Ranunculus repens was found seldom at half dosage compared to plots sprayed with quarter or normal dosage; and Atriplex patula was found seldom at normal dosage compared to reduced dosages. Only one of the species was found exclusively at one dosage: barley as weed was only present in half dosage plots.
Rare species
None of the species found in this experiment is mentioned in the Danish Red List (Stoltze & Pihl 1998). However, a few of the agricultural weed species found are quite rare in the Northern and the Western parts of Denmark according to Hansen (1981) and Mikkelsen (1989) viz. Chaenorhinum minus, Euphorbia exigua, Kickxia elatine, Silene noctiflora, Stachys arvensis and Veronica hederifolia. Table 2.4 shows the number of plots where the six species were observed at least once during the three years of study. A species was only counted once in each plot to avoid dependent observations, coming from identical populations year after year.
Table 2.4.
Number of plots where six rare species occurred at least once during the
three years.
Species |
Dosage |
||
Normal |
Half |
Quarter |
|
Chaenorhinum minus |
0 |
2 |
0 |
Euphorbia exigua |
4 |
6 |
7 |
Kickxia elatine |
3 |
3 |
4 |
Silene noctiflora |
6 |
7 |
5 |
Stachys arvensis |
1 |
1 |
3 |
Veronica hederifolia |
0 |
1 |
0 |
The occurrence of the rare species was not significantly influenced by differences in dosages (chi2-test, df=6 (the three species with low occurrence was summed), p>0.1).
Scarce species
The scarce species (found in less than 6 of the 123 investigated plots) can be divided into different groups based on habitat preferences:
Woodland species: Acer pseudoplatanus, Fraxinus excelsior, Salix sp. and Sambucus nigra.
Crop species: Beta vulgaris ssp. vulgaris, Hordeum vulgare, Medicago lupulina and Secale cereale.
Species from roadsides and meadows: Achillea millefolium, Artemisia vulgaris, Carduus crispus, Cerastium fontanum ssp. triviale, Cirsium vulgare, Festuca rubra, Ranunculus acris ssp. acris and Rumex crispus.
Species growing in dry or wet soils: Arabidopsis thaliana, Arenaria serpyllifolia, Bidens tripartita, Epilobium parviflorum, Filaginella uliginosa and Juncus bufonius.
Arable species: Alopecurus myosuroides, Avena fatua, Chaenorhinum minus, Galeopsis tetrahit, Geranium pusillum, Papaver dubium, Papaver rhoeas, Raphanus raphanistrum, Stachys arvensis, Thlaspi arvense, Veronica hederifolia and Viola tricolor ssp. tricolor.
Table 2.5.
Number of plots where at least one of the species in a group has appeared, at
least once in three years.
Group |
Dosage |
||
Normal |
Half |
Quarter |
|
Woodland species |
4 |
0 |
3 |
Crop species |
1 |
5 |
2 |
Species from roadsides and meadows |
3 |
1 |
9 |
Species growing in dry or wet soils |
1 |
2 |
4 |
Arable species |
1 |
7 |
8 |
A higher number of scarce species were found at quarter dosage than at normal and half dosages. This has to be seen in connection with the general higher species richness at quarter dosage than at normal dosage. However, the relative proportion of scarce species might be higher at quarter dosage than at normal dosage.
There was a significant effect of dosage on the group occurrences (chi2-test, df=8, 0.01<p<0.05). A higher occurrence of woodland species than expected was found at normal dosage, moreover the occurrence of crop species was higher than expected in plots sprayed with half dosage (Table 2.5).
The occurrence of woodland species at normal and quarter dosages and not at half dosages might be explained by the experimental design, since a higher proportion of plots receiving normal and quarter dosage were located near hedges (see section 1.2.5). The dispersal of seeds from woody species is expected to be more frequent near hedges. In contrast, crop species occurred most often in the half dosage plots more distant from hedges.
2.2.2.4 Ability to flower
Proportion of flowering plants
The proportion of flowering plants was on average 44 % ranging from 5 % to 76 %. Fig. 2.12 illustrates the proportion of flowering plants in relation to crop and dosage. Table 2.6 lists the result of the variance analysis for proportion of flowering plants.
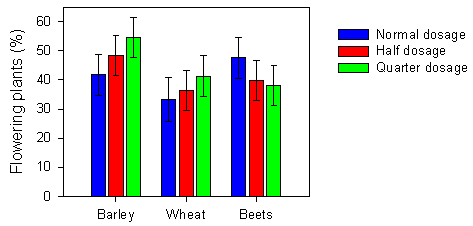
Fig. 2.12.
Mean proportion of flowering plants after spraying with normal, half or
quarter dosage of pesticides in three crops. Each bar represents a mean of 8-10 values.
The error bars indicate the 95 % confidence limits.
A significant effect of dosage was found in the analysis of the proportion of flowering plants, but it varied between crops (Table 2.6). In the cereals, the proportion of flowering plants increased with decreasing dosage. In contrast, the proportion of flowering plants in the sugar beet fields decreased with decreasing pesticide dosage (see sections 2.2.2.5 and 2.2.3.5 for further explanation). Moreover, the proportion of flowering plants was highly affected by the variation between fields.
Proportion of flowering species
The proportion of species recorded flowering was on average 62 % but varied between 17 % and 100 %.
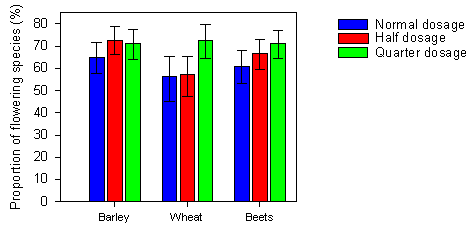
Fig. 2.13.
Mean proportion of flowering species after spraying with normal, half or
quarter dosages of pesticides in three crops. Each bar represents a mean of 8-10 values.
The error bars indicate the 95 % confidence limits.
Dosage had a highly significant effect on the proportion of flowering species in a plot (Table 2.6 and Fig. 2.13). The proportion of flowering species was significantly higher at quarter dosage than at normal dosage (p=0.0032). Moreover, the proportion of flowering plants affected the proportion of flowering species positively. More plants in flower increased the possibility that a higher proportion of different species was represented.
Table 2.6.
Schematic summary of the analyses of proportion of flowering plants and
flowering species, respectively. Factors not included in any of the models have been
omitted from the table. Statistical significance is indicated as follows: +: 0.05£ p<0.10, *: 0.01£ p<0.05, **:
0.001£ p<0.01 and ***: p<0.001.
Explanatory factors |
Ability to flower |
|
Plants (n=83) |
Species (n=82) |
|
Dosage |
|
** |
Proportion of flowering plants |
|
** |
Crop |
* |
+ |
Farm |
|
* |
Field(Farm) |
*** |
|
Dosage´ Crop |
* |
|
2.2.2.5 Habitats in sugar beets
Fig. 2.14 shows occurrence of weeds in the sugar beet fields grouped by their habitat: within the rows or between the rows.
Total weed density and species richness in sugar beets were not significantly influenced by the habitat (Fig. 2.14A and C) (anovas, p>0.1). Nevertheless, both were strongly affected by dosage (see sections 2.2.2.1 and 2.2.2.2).
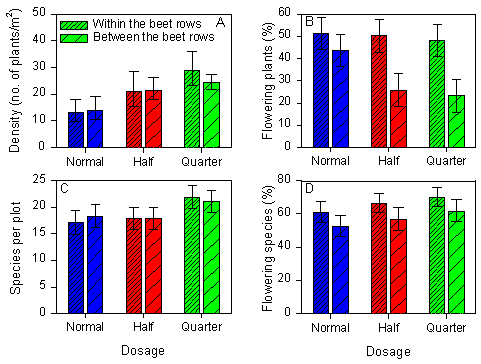
Fig. 2.14.
A) Total weed density, B) proportion of flowering individuals, C) number of
species per plot and D) proportion of flowering species in sugar beet fields after
spraying with normal or reduced dosages of pesticides. For each dosage the weed plants are
grouped by their habitat in the field: within the beet rows (dense hatching) or between
the beet rows (light hatching). Each bar represents a mean of ten plots and error bars
indicate the 95 % confidence limits.
The proportion of plants flowering was significantly affected by habitat, although the effect varied between dosages (p=0.036 for the dosage´ habitat interaction). The response of habitat was strongest at the reduced dosages (Fig. 2.14B). No significant effect of dosage was found on proportion of flowering plants within the beet rows, whereas between the rows 44 % of the weed plants were flowering at normal dosage, 26 % at half dosage and only 23% at quarter dosage.
Equally, the proportion of flowering species was close to significantly affected by habitat (anova, p=0.074), with more species capable of flowering within rows than between rows (Fig. 2.14D).
2.2.3 Discussion
2.2.3.1 Weed density
Dosage effect
Investigation of the effect of pesticide application on weed densities showed a reduction of 45 - 70 % after spraying (Fig. 2.8), dependent on the dosage applied. This reduction can be related to the effects of the herbicides and to other natural changes in the population occurring between the time of spraying and the registration three months later. This period covers most of the growing season for annual plants, in which some seeds still germinate, seedlings become established, plants flower and set seeds, if the conditions allow it. In addition, many of the seedlings could have died as an effect of intra- or interspecific competition. The yield trials in this study have shown that the densities of weed plants in unsprayed areas of spring barley decreased with 6 % from seedlings to mature plants, whereas the densities in areas sprayed with quarter dosage decreased with 25 % (see section 5.1). Therefore, the reductions in weed densities are mainly due to the herbicide sprayings. No significant differences in weed densities between half and quarter dosages were found, whereas the density at normal dosage was significantly lower.
The biomass reduction after spraying is often higher than the density reduction (Salonen 1993a) indicating that the growth of each surviving plant may be reduced too. Therefore the relationship between weed density and weed biomass is probably dosage-dependent, making density just a rough measure of biomass.
For four of the twelve most dominant species (Aethusa cynapium, Bilderdykia convolvulus, Lamium sp. and Stellaria media), it was possible to show a significant effect of dosage, with fewer plants killed at quarter dosage than at normal dosage (Table 2.2). The responding species were both target and non-target species, indicating that densities of both categories increased at strongly reduced pesticide dosages. Thus, even though, the herbicides were chosen mainly to control the target species, densities of non-target species also decreased at spraying with normal dosage.
Elymus repens is a weed species farmers want to control, but most of the herbicides used in spring against broad-leaved species are not effective against grasses. E. repens is usually controlled by glyphosate in the autumn. Thus, E. repens and Poa annua do not show any significant dosage response to herbicides used in spring. The rare non-target species Silene noctiflora and Euphorbia exigua were negatively influenced by the dosage of herbicides used (Fig. 2.9) though only significant for Euphotbia exigua in two of three years. Many herbicides are broad-spectrum herbicides, affecting the density of target species as well as non-target species, and may result in a local or temporary loss of non-target species (McLaughlin & Mineau 1995), as was confirmed by this study. Use of reduced herbicides dosages may increase the population size and thus improve possibilities of long-term survival in the arable fields.
The different interspecific responses observed at reduced dosages might be due to different susceptibility towards the herbicides (e.g. Cashmore & Caseley 1995).
This study covers very different herbicide products and spraying situations, which increases the statistical uncertainty. However, the results include the great variations found between farms and years and may therefore be of more general value, than studies of responses to one herbicide on one farm in one year.
Many dose response trials have been performed, where plants of one weed species have been treated with many different dosages of one herbicide and the biomass measured afterwards, resulting in a mathematically described dose response curve (e.g Streibig 1992, Streibig et al. 1993, Olofsdotter et al. 1994). Despite only three points on a dose response curve were revealed in this study, it is the first time that large-scale dose response trials have been performed over different weed communities of several weed species, different herbicide products and different years, resulting in much variation. Therefore it is without much value to compare directly with known dose response trials.
Time effect
The increase in weed densities after spraying over the three years is difficult to explain but might reflect variations in growing and spraying conditions across the different years, resulting in a higher percentage of individuals surviving at all dosages in 1999 than in 1997. Populations of short-lived plant species often vary in number of individuals between years (e.g. Milberg et al. 2000). Moreover, reductions in total weed number caused by herbicide application may vary considerably. One year Derksen et al. (1995) found a 90 % reduction in total weed number due to a herbicide spraying, next year the reduction was only 39 % despite the field, the product and the dosage being similar.Accumulation of dosage effects are described in section 2.3.3.1.
2.2.3.2 Species richness
Crop effect
This study has found a higher species richness in spring barley than in winter wheat (Fig. 2.10). The spring cereals in Denmark have more weed species than winter cereals (Andreasen et al. 1996, Hald 1999), because the weed flora in Denmark has been selected over many years towards the ecological conditions prevailing in spring sown crops (Hald 1999). This is confirmed by the dominance of pure summer annuals in spring barley and by the fact that all except one of the winter annual species present in this study were also capable of germination in spring. Eight of the eleven winter wheat fields sprayed in spring were also sprayed in autumn. The number of species was thus reduced twice, which might be another reason for the lower species number in winter wheat than in spring barley. Furthermore, winter cereals have a higher degree of cover at springtime than spring cereals. The competition in spring from the crop against the weed species is thus stronger in winter cereals than in spring cereals.
Dosage effect
Species richness was affected by the dosage of pesticides used; the lowest dosage gave the highest species richness (Fig. 2.10). A reduction in pesticide dosage from normal to quarter dosage resulted in 28 % more species, and a reduction to half dosage resulted in 16 % more species on average over all three crops. Thus, reduced pesticide input promotes higher weed diversity as suggested by Clements et al. (1994). The increase in richness at reduced dosages was not solely an effect arising from the fact that species diversity increases with an increase in plant density (the species-area relationship). This effect was accounted for in the analyses by including weed density as a covariate (with a significant and positive effect on richness). To sum up, dosage affects richness both directly and indirectly through density. A total cease of herbicide use would presumably increase the richness even more as found by Boström & Fogelfors (1999). In this experiment, knowledge of the size of increase in weed richness with no use of herbicides would have allowed us to conclude whether a 28 % increase was high compared to the maximal possible.
It is worth noticing, that it was possible to detect an increased species richness at reduced pesticide dosages, even though the arable fields are poor in plant richness and the potential species pool has been strongly diminished the last decades (Jensen & Kjellsson 1995) and there were large variations in weed communities between fields.
Time effect
No clear tendencies towards an increase in species richness could be detected between years, even after two subsequent years with reduced dosages of herbicides (Fig. 2.11).
2.2.2.3 Species composition
This study has like others (Andreasen et al. 1991, Wilson et al. 1994) demonstrated that species abundance and species composition also vary considerably from field to field. Despite the fact that all fields are placed on clay soils in the same region in Denmark. Differences between fields may be the result of different agricultural histories before the start of the main experiment and different agricultural practices during the study years, including among other things differences in the pesticide products used and differences in the dosage chosen as normal.
It proved impossible to find indicator species in the sense, that the species exclusively occurred in fields receiving only reduced dosages of pesticides, due to the huge variation in dominant weed species from field to field. Scarce species were found more often at quarter than at normal dosage, which may be due to a higher possibility of being established in quarter dosage plots as seeds from hedges and habitat islands.
2.2.3.4 Ability to flower
No general effect of dosage was seen on the proportion of flowering plants, but dosage had different effects on the ability to flower in cereals and in sugar beets. The expected inverse relationship between proportion of flowering species and dosages was seen in cereals, whereas in sugar beets the proportion of flowering plants was higher at normal than at reduced dosages (Fig. 2.12). This was a result of the mechanical control of weeds between the beet rows (see section 2.2.3.5). A higher proportion of flowering plants at the reduced dosages in cereals is probably accompanied by a higher plant biomass on average, since flowering annual plants has more biomass than vegetative annual plants - in general. Debaeke (1988) showed a positive relationship between dry weight per plant and number of seeds produced per plant. Therefore, it is possible that reduced pesticide dosages will result in a higher seed production per surviving plant as shown for some species (Hald 1993, Rasmussen 1993a, Rasmussen 1993b).
Dosage had a strong impact of the proportion of flowering species (Fig. 2.13), both directly and indirectly through the proportion of flowering plants, resulting in more reproductive species at quarter dosage than at normal dosage. This is to our knowledge the first time it has been demonstrated that reduced pesticide dosages increase a weed community's ability to flower. These results imply that the fitness of surviving plants is higher for plants exposed to reduced dosages than for plants exposed to normal dosage.
2.2.3.5 Habitats in sugar beets
Differences between the two habitats for weeds in the sugar beet fields had a strong impact on the proportion of flowering plants. Hoeing between the rows reduced the proportion of flowering plants with more than 50 % compared to plants exposed to spraying within the beet row. Although the density of plants was not significantly different between habitats, the ability to flower was highly affected. Hoeing operations were often performed later in the growing seasons than the sprayings, and harmed plants at a higher developmental stage. In addition, hoeing dried out the roots of weed plants between rows and promoted new weed seedlings to germinate. Most of these seedlings had not the time to reach flowering and could therefore never set seeds. Although plants are very plastic, they need time to reach a size where the plant has the energy necessary for flowering. This time is longer after spraying than after hoeing, because hoeing is performed later in the growing season than spraying. Hoeing in combination with 25 cm band spraying compared with broad spraying have not increased the weed density significantly, whereas 12.5 cm band spraying in combination with hoeing resulted in significantly higher weed density than at broad spraying (Fig. 2.14). The hoeing in contrast to the spraying gives each surviving weed plants a lower ability to flower than at broad spraying, probably resulting in a lower seed set. However, the overall proportion of species flowering was still higher in low dosage plots than at normal broad-spraying.
2.3 Seed rain study
In this study, the term seed rain refers to seeds lying on the soil surface called surface seed bank by Mortimer (1976). The aim of the study was to measure the effect of reduced pesticide dosages on the seed number per square meter soil surface, the diversity of species (richness) and the seed biomass available for bird consumption after crop harvest. The seed rain is not only eaten by birds (e.g. Christensen et al. 1996), but also insects (Van der Wolf 1992, Cromar et al. 1999) and small mammals like mice (Green 1979, Angelstam et al. 1987) utilise the seeds as a food resource.
It may be assumed that all seeds constitute a potential food resource for e.g. birds and mice, but seeds of some weed species might be poisonous or be avoided for other reasons (Diaz 1990). The seed rain may also play a role as food resource after the seeds have germinated and become seedlings (e.g. Green 1980). Especially in winter, seeds on arable stubble fields constitute a major part of the food eaten by birds in the agricultural landscape (Steenfeldt et al. 1991, Donald et al. 2001). The number, composition and richness of seeds in the seed rain are very important for the structure of the vegetation in the following years. Most of the seeds enter the soil seed bank as a result of cultivation and then become a part of the potential future weed vegetation.
2.3.1 Methods
2.3.1.1 Field work
The seeds on the five winter wheat and the five spring barley stubble fields were sampled in autumn of 1998 and 1999, where bird counts were carried out (section 4.2.2). High precipitation made it impossible to collect seeds from the winter wheat stubble field at Gjorslev in 1998; therefore, no data exist from that field. Unfortunately, one farmer did not wish to continue the investigations on the stubble fields in 1999. Thus, 9 fields were investigated in 1998 and 8 fields in 1999.
The seed rain was sampled once every year, on average 12 days after harvest, depending on the weather and the time of straw collection. The samples were taken in dry and sunny weather, after the dew had evaporated, and at least five hours after rainfall. All samples from one field were taken on the same day. At each field 12 samples (4 per plot at regular intervals) from 0.18 m2 were taken with a C-vac constructed by Navntoft et al. (see Fig. 3.2). Each sample was taken as ten 5-seconds suctions covering 0.018m2. The samples consisted of surface soil, seeds, straw, awns and seed shells from the cereals. An average sample weighed 34 g of which less than 0.62 g were weed seeds. The samples were taken at least 12 meters from other plots, hedges etc. to avoid edge effects.
2.3.1.2 Laboratory work
In the laboratory, the samples were air dried at 20 oC, to avoid seed germination and seed predation by insects present in the samples. The dry samples were fractionated using a 2-mm and a 0.5-mm mesh sieve successively. Every organic particle over 2 mm was manually sorted into seeds or debris. Soil clumps bigger than 2 mm were manually pushed through the mesh. Particles less than 0.5 mm were dropped to reduce the bulk and thereby save time in the laboratory. This was done with two arguments: 1) The vast majority of weed seeds have a minimum diameter bigger than 0.5 mm in diameter (Holm-Nielsen 1998) and none of the dominant weed species present in this study had seeds that small. 2) Seeds smaller than 0.5 mm in diameter are only eaten by very few bird species foraging in the agricultural land (Christensen et al. 1996). Species with small seeds are, however, very important with respect to other aspects of the vegetation dynamics.
After sieving, the samples were weighed and submersed in a flotation solution of potassium carbonate (K2CO3) with a specific density of 1.43 g/ml. Specific densities of weed seeds varied between species from less than 0.7 to 1.42 g/ml (Jensen unpublished data). The amount of flotation solution was between 1 and 2 times the volume of the seed samples. After 24 hours, the high-density particles precipitated and the low-density particles (organic material) remained at the surface. The supernatant was carefully transferred to a filter paper over a vacuum pump, which removed the remaining flotation solution. The filter paper with all the organic material was placed in a petri dish and air-dried at 20 oC. Then the material was spread in a thin layer under a magnifying stereoscope and all seeds and seed shells were identified to species and counted. Seeds were identified by literature (Beijerinck 1947, Holm-Nielsen 1998) or by comparison with a seed reference collection made from mature plants in the fields. Some seeds were only identified to genus level, e.g. seeds from Lamium amplexicaule, Lamium hybridum and Lamium purpureum were very difficult to distinguish from each other (pers. obs.), thus seeds from these species were all called Lamium sp. Seeds from Atriplex patula and Chenopodium album looked like each other and were treated as one species: Atriplex patula/Chenopodium album. A few seeds were impossible to identify although they were clearly different from all other seeds. Those seeds were named type A.
2.3.1.3 Data description
The seeds were divided in two groups: whole seeds and damaged seeds. Seeds resisting the pressure from a pair of tweezers were registered as whole seeds. Seeds not resisting the pressure were hollow and thus categorised as damaged seeds together with pieces of seeds and seed shells. It was attempted to estimate how many whole seeds the pieces of seeds and seed shells in the samples corresponded to, and this figure was added to the hollow seed count. The number of damaged seeds calculated in this way was a conservative measure, because small pieces of seeds and seed shells might be lost during sieving.
For comparison with bird data, seeds were divided in two groups: Spilt grains and weed seeds. For each group, the number of seeds and the biomass per square meter were calculated. The mean seed weight of most species is known from the literature (Korsmo 1926, Salisbury 1942, Gross 1990, Melander 1993) (see Appendix C.2). If there was great variation between seed weight mentioned by different authors or no seed weight could be found in the literature, seeds of those species were weighed in the laboratory. The total biomass was calculated by multiplying the seed weight of each species with the number of seeds per species and adding the biomass for all species in a sample. The same seed weight was used for whole and damaged seeds, although most of the damaged seeds weighed less than a whole seed. Number of weed species was counted as the number of taxa in a plot, excluding taxa on genus or family level in which a taxon at species level was counted.
2.3.1.4 Control of methods
26 of 204 seed samples selected at random were checked for viable seeds in the discarded sample parts. There were several discarded parts: 1) Organic material over 2 mm classified as debris. 2) Sediment from the flotation. 3) Residuals from the petri dish after visible seeds had been picked out. All discarded parts were placed in a tray on a substrate of sterilised soil and placed in a greenhouse for three months to see if any germination occurred. Seedlings were counted and identified to species. The greenhouse was different from the greenhouse used in the pilot study (section 2.1) and the seed influx was nearly zero.
2.3.1.5 Statistical analyses
The following response variables were analysed statistically:
- Mean number of weed seeds per m2
- Mean biomass of weed seeds per m2
- Mean number of spilt grains per m2
- Mean biomass of spilt grains per m2
- Number of weed species per plot
- Proportion of damaged spilt grains
- Proportion of damaged weed seeds
- Proportion of damaged seeds per species
The basic experimental unit in the analyses of all variables was the plot (n=51). In order to improve approximation to a normal distribution and make the variance independent of the mean, the mean number of seeds and seed biomass (variables 1-4) were loge (y+1) transformed before further analyse. The proportions of damaged grains (variable 6) were square root transformed and the proportions of damaged weed seed (variable 7) were log (arcsine (y)) transformed. The data transformations were accepted, if after running the model the residuals plotted against the predicted values were without apparent trends.
The proportion of damaged seeds was calculated as the number of damaged seeds divided by the number of damaged and whole seeds per plot. Data for variables 1-5 included damaged seeds. The factors used to explain the variation in seed rain were: dosage, crop, farm, year, density of generative plants, the sample weight and the interactions dosage´ crop, dosage´ farm, dosage´ year, crop´ year, dosage´ crop´ year and crop´ farm´ year. The log-transformed sample weights were used in the analyses, to allow for possible effects of sample size on the number of seeds found.
Response variables 1-7 were analysed separately in a general linear model with the explanatory factors mentioned above. The number of explanatory factors was reduced during the full-scale model calculations using an iterative procedure removing the variables with p > 0.1 until the model consisted of variables with p £ 0.10 only. The analyses were performed using the GLM procedure (with Random/Test statement) in SAS (SAS Institute 1999).
The proportion of damaged seeds of a given species (variable 8) was tested with a Kruskal-Wallis test with regard to differences in median values between dosages. Only plots with more than ten seeds of a given species were tested, because a rather large stochastic variation exists in proportions calculated from small populations.
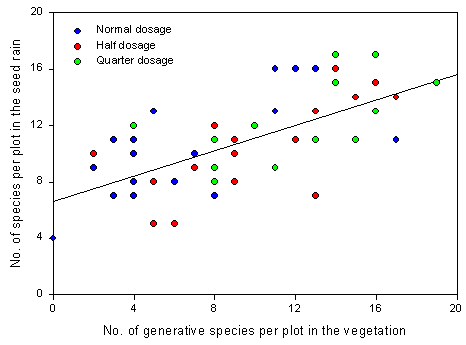
2.3.2 Results
2.3.2.1 Seed number and biomass
The seed rain of the spring barley and winter wheat stubble fields consisted of spilt grains and weed seeds. On the 17 investigated fields there were on average 62 spilt grains (corresponding to 1.77 g dry weight) and 206 weed seeds (corresponding to 0.18 g dry weight) per m2. Even though there were three times as many weed seeds than spilt grains per m2, the weed seeds made up less than 10 % of the total seed rain biomass (Fig. 2.15). A total of 19,980 weed seeds and 7,920 spilt grains were found (Appendix C.2).
Weed seeds
The main contributors to the seed rain were Stellaria media, Atriplex patula/Chenopodium album, Aethusa cynapium and Polygonum aviculare, which together accounted for 46 % of the seeds. Bilderdykia convolvulus made up the largest proportion of the weed seed biomass (24 %) followed by Stellaria media and Atriplex patula/Chenopodium album each with 12 %.
Look here!Fig. 2.15.
Seed rain in 17 stubble fields at three dosages of pesticides. A) shows the
geometric means of the number of spilt grains and weed seeds (per m2),
respectively. B) shows the geometric means of the biomass of spilt grains and weed seeds.
Error bars represent the 95 % confidence limits. Damaged seeds were included in the
estimates.
The number and biomass of weed seeds was not significantly affected by pesticide dosage (Fig. 2.15 and Table 2.7), though it appeared higher at quarter dosage than at normal dosage with half dosage in between. The number of weed seeds per m2 ranged from 9 to 2779 across dosages, crops and years. The covariate (density of generative plants in the vegetation) was highly significant in the analysis of seed number and biomass and explained one third of the variation in both response variables. If the covariate was excluded from the model, dosage had still no significant effect on the weed seeds. The analysis showed that there were significantly more weed seeds present in winter wheat fields than in spring barley fields. The farm factor had a significant effect on the weed seed number, due to the occurrence of significantly more seeds on Nøbøllegård than on the other farms (Tukey-Kramer tests). There were especially many seeds of Matricaria perforata on Nøbøllegård. Matricaria perforata has light seeds (Appendix C.2), which may explain why the effect of farm was not as strong in the analysis of biomass (Table 2.7).
Table 2.7.
Schematic summary of the analyses of the seed rain. Factors not included in
any of the reduced models have been omitted from the table. Statistical significance is
indicated as follows: +: 0.05£ p<0.10, *: 0.01£ p<0.05, **: 0.001£ p<0.01 and
***: p<0.001. Grey areas indicate explanatory factors not included in the full model.
|
Weed seeds |
Spilt grains |
|||
|
no/m2 |
mg/m2 |
no/m2 |
mg/m2 |
no/m2 |
Sample weight |
|
|
* |
+ |
|
Density of generative plants |
*** |
*** |
|
|
|
Dosage |
|
|
** |
* |
|
Crop |
** |
* |
|
|
|
Farm |
*** |
** |
|
|
|
Dosage´ Crop´ Year |
|
|
|
|
** |
Crop´ Farm´ Year |
|
|
*** |
*** |
*** |
Spilt grains
Reduction of pesticide dosage affected the number and biomass of spilt grains significantly (Table 2.7). There was an estimated geometric mean of 45 grains per m2 in plots sprayed with normal dosage compared to 82 grains per m2 in plots sprayed with half or quarter dosages (Tukey-Kramer test, p=0.009 and p=0.011, respectively).
In 1998, two of the fields were sprayed with glyphosate before harvest (Appendices A.3 and A.4). To detect if the number of spilt grains was influenced by dosage of Roundup sprayed on the fields before harvest, the analysis was run once more without data from these two fields. The dosage had no longer any general significant effect on the number of spilt grains on the remaining fields (analysis of variance, p=0.43). However, the interaction between dosage, crop and year was significant in the model indicating that there were still differences between dosages that varied between years and crops (at the remaining barley fields, the number of grains was lowest at quarter dosage in 1998, while in 1999 the highest number of grains was also found at quarter dosage).
The number and biomass of spilt grains was also affected by the interaction between crop, farm and year (Table 2.7) explaining more than 70 % of the variation in number and biomass of spilt grains. Furthermore a positive correlation between sample weight and number of spilt grains was found, but the sample weight explained less than 2 % of the variation in number of spilt grains.
2.3.2.2 Species richness
A total of 39 weed species were found in the seed rain (Appendix C.2). There were between 4-17 species per plot. On average 11 species were present per plot sprayed with quarter or normal dosages and 10 species per half dosage plot.
Dosage had no significant effect on the number of species per plot (Table 2.8), and the significant explanatory factors were number of generative species per plot, farm and the interaction between year and crop.
Table 2.8.
Schematic summary of the analysis of weed richness in the seed rain.
Factors not include in the reduced model have been omitted from the table.
Statistical significance is indicated as follows:
*: 0.01£ p<0.05, **: 0.001£
p<0.01 and ***: p<0.001.
Factors |
Number of weed species per plot |
No. of generative species per plot |
*** |
Farm |
** |
Crop´ Year |
* |
The number of species per plot in the seed rain was positively correlated with the number of generative species in the vegetation (Fig. 2.16). The correlation explained 11 % of the total variation in number of species per plot in the seed rain.
The differences among farms in species richness were mainly caused by a significantly lower species richness per plot on Gjorslev (9.1 species) and Lekkende (8.9 species) than at Nøbøllegård (12.8 species).
Fig. 2.16.
Correlation between the number of species found in the seed rain and the
number of generative species in the vegetation. The relation did not vary significantly
between dosages (p=0.33 in test for homogeneity of slopes).
The effect of crop ´ year showed that there was a higher species richness in spring barley fields than in winter wheat fields in 1998, and a higher number in winter winter wheat than in spring barley in 1999. Because of the crop rotation, the fields with spring barley in 1998 were exactly the same fields as the fields with winter wheat in 1999.
Comparing species richness in seed rain and vegetation
A total of 61 species were found in the vegetation study on the 17 seed rain fields, whereas only 39 species were found in the seed rain study. Of this, 36 species were found in both studies. Some of the seeds could not be identified to species but only to genus, therefore the number of species present was probably higher than indicated (Table 2.9).
Table 2.9.
Number of species found in the vegetation in July and / or in the seed rain
in September.
Number of species |
In seed rain |
Included in seed rain on genus level |
Not in seed rain |
Total |
Generative in vegetation |
32 |
7 |
8 |
47 |
Vegetative in vegetation |
4 |
1 |
9 |
14 |
Not in vegetation |
3 |
0 |
- |
3 |
Total number |
39 |
8 |
17 |
64 |
2.3.2.3 Damaged seeds
To detect if dosage had an effect on the proportion of damaged seeds a variance analysis was performed on the proportion of damaged weed seeds and damaged spilt grains. The mean proportion of damaged seeds (22 %) and spilt grains (24 %) were almost identical (Fig. 2.17). Most damaged grains were pieces of grains, whereas most of the damaged weed seeds consisted of hollow seeds and to lesser extent of intact seeds and seed shells.
Fig. 2.17.
Mean proportion of damaged seeds (hollow seeds + seed shells + pieces of
seeds) at different dosages of pesticides. Error bars represent the 95 % confidence
limits.
Dosage did not have any general significant effect on the proportion of damaged spilt grains. Only the interaction between farm, year and crop could explain a significant part of the variation in proportion of damaged spilt grain (Table 2.10).
The proportion of damaged seeds varied between species from zero in Galium aparine and Veronica agrestis/persica to 90 % in Atriplex patula/Chenopodium album. When the proportion of damaged seeds was analysed no significant effect of dosage was seen in the nine most abundant species (Kruskal-Wallis and Wilcoxon tests, 7<n<28, p>0.10 in all nine cases).
The effect of pesticide dosage on the proportion of damaged weed seeds varied between farms. On three farms, the proportion of damaged weed seeds was highest at normal dosage, while the remaining two farms had the highest proportions either at half dosage or quarter dosage. Furthermore, the farm factor had a significant effect on the proportion of damaged weed seeds. Two farms (Nøbøllegård and Gjorslev) had a very low proportion of damaged seeds (10 %), while the three other farms had a high proportion of damaged seeds (42 %). In this context, it is interesting that different weed species were dominant in the seed rain at different farms. By number, Matricaria perforata dominated on Nøbøllegård and Aethusa cynapium dominated on Gjorslev and both Matricaria perforata and Aethusa cynapium had a very low proportion of damaged seeds.
Table 2.10.
Schematic summary of the analyses of proportion of damaged seeds in the seed
rain. Factors not included in any of the reduced models have been omitted from the table.
Statistical significance is indicated as follows: +: 0.05£
p<0.10, *: 0.01£ p<0.05, **: 0.001£
p<0.01 and ***: p<0.001.
|
Proportion of damaged seeds |
|
|
Spilt grains (n=51) |
Weed seeds (n=51) |
Farm |
|
* |
Year |
|
+ |
Dosage´ Farm |
|
* |
Crop´ Farm´ Year |
*** |
** |
2.3.2.4 Control of method
From the trays with discarded parts 78 seeds germinated. In the same 26 samples 2706 whole seeds were found under the stereoscope after flotation (Table 2.11).
Table 2.11.
Total number of whole seeds in the 26 control field samples found under
stereoscope or in the discarded parts by germination. - : Indicates that the material has
not been investigated.
Parts |
Weed seeds |
Grains |
||
Number of whole seeds found in samples or in discarded parts |
found |
discarded |
found |
discarded |
1) Discarded organic material over 2 mm from sieving before flotation |
23 |
20 |
837 |
22 |
2) Discarded sediment from the flotation |
- |
0 |
- |
0 |
3) Discarded material in petri dish after visible seeds have been picked out |
1808 |
33 |
38 |
3 |
Effectiveness of methods used |
97 % |
97% |
||
The flotation method gave a success rate around 97 % for both weed seeds and spilt grains extracted from a sample. No germination occurred in the sediment from the flotation. This might be due to the fact, that there were no seeds in the sediment, but more likely, that some remaining potassium carbonate might have prevented germination (Tsuyuzaki 1993). Thus, the actual success rate may be lower than indicated.
2.3.3 Discussion
2.3.3.1 Seed number, biomass and species richness
Weeds
No direct effect of dosage was found on the number of seeds, biomass or richness. However, a highly significant correlation was found between the density of generative species in the vegetation and the biomass and number of seeds. In addition, the number of generative species in the vegetation was positively correlated with the species richness in the seed rain (Fig. 2.16). Both weed density and species richness in the vegetation were highly affected by dosage (sections 2.2.2.1 and 2.2.2.2); so reduced dosages have indirectly a positive effect on the seed rain. The great variation found between farms makes it very difficult to detect dosage differences.
No clear tendency towards an increase in germination of weeds could be detected, even in the third year with quarter dosage of herbicides (Fig. 2.8). Ploughing turns around the soil, so seed rain from the first year may not reach the soil surface before spring the third year. These findings are in accordance with Salonen (1993c), who did not find any increase in weed densities during four years without the use of herbicides. The soil seed bank may work as a buffer (Wilson & Lawson 1992), so it takes several years before a possible increase in germination can be detected. A continuous reduction of inputs of herbicides over a period of years has been found to result in an increase of seeds from weedy species accumulating in the soil seed bank, followed by an increased weed problem in subsequent crops (Hill et al. 1989). However, this study does not support those results. No significant response can be due to the low density of weeds at the beginning of the experiment and the lack of problematic grass weed species. In a longer period with use of reduced dosages the effect may be more pronounced. An experiment with low inputs of herbicides through 6 years compared to recommended inputs of herbicides revealed a significant positive effect on the number of seeds in the soil seed bank on one of two farms (Jones et al. 1997).
The higher density of seeds found on winter wheat stubble fields than on spring barley stubble fields may be due to a longer growing season in winter cereals than in spring cereals, especially for annuals germinating in autumn and winter. Thus, weed plants in winter cereals may have a higher biomass when they reach the seed setting phase and may then be capable of a higher seed set (Thompson et al. 1991).
A weed seed rain of approximately 200 seeds per m2 right after crop harvest may seem rather low in comparison to soil seed bank estimates of 128,000 seeds per m2 in fields in Denmark (Jensen & Kjellsson 1995). However, if seeds of Juncus bufonius are excluded from this figure the soil seed bank estimate becomes only 12.000 per m2 (Jensen & Kjellsson 1995). The use of geometric mean (this study) compared to arithmetic mean (Jensen & Kjellsson 1995) make the difference seems larger than it is. The soil seed bank constitutes seed rain from several decades why the content of soil seed banks might be expected to be much higher than the seed rain from one year. In the same period, the farmers have intensified their use of herbicides considerably, their use of more competitive crop cultivars, their use of more "effective" ploughs etc. resulting in a general low weed density (Andreasen et al. 1996). It must be assumed that most seeds in the seed bank are very old, which the low seed viability found by Jensen & Kjellsson (1995) infers.
Grains
The dosage effect on number of spilt grains was only observed in fields sprayed with Roundup a few weeks before harvest. The number of spilt grains per m2 was lowest in plots sprayed with normal dosage of Roundup. This could be explained by at least two factors, maybe in combination: 1) A homogenous ripening of grains at normal dosages of Roundup, but not at reduced dosages. This may result in lowest escape during harvest at normal dosage because the size and hardness of the grains will be more homogenous. 2) Reduced dosages could result in a higher green weed biomass, which could reduce the effectiveness of the combine harvester and result in a higher amount of spilt grains. Sheppard et al. (1984) showed that pre-harvest spraying with Roundup compared to no spraying resulted in lower moisture content of the grains and lower grain loss from the combine harvester. This might be the case with reduced dosages too, however more experiments are needed before clear conclusions can be drawn.
2.3.3.2 Damaged seeds
This study has shown that a least 24 % of the weed seeds shed in a particular year are hollow or so badly damaged that they are not viable. Investigations of the soil seed bank in Western Denmark (Jensen 1969, Jensen & Kjellsson 1995) have also revealed a very high proportion of non-viable seeds (73 % and 79 %). The pool of dead seeds in the soil seed bank consist of both hollow seeds and whole seeds not capable of germinating, in comparison to the 24 % damaged seeds in this study, that mostly consisted of hollow seeds. The whole seeds found in this study have not been tested for viability, and many might be non-viable. In addition, some seed shells are very robust and might persist for years in the soil seed bank before decomposition although the embryo is dead (Roberts & Ricketts 1979).
On three of five farms, the ratio of damaged seeds was highest at normal dosage. This result might arise by chance or be due to the reduction in herbicide dosages. Surviving plants at normal dosage might be more stressed and more delayed in development than at quarter dosage thus having less energy resources to complete seed formation and a degeneration of the embryo and the endosperm takes place. The existence of huge differences in ratios of damaged seeds between weed species is interesting - a high production of hollow seeds (empty seed shells) seems a waste of energy. Abortion of seeds was expected to happen when seeds were immature and before the seed shells were totally developed and looked 100 % like mature seeds. A plausible explanation for a high percent of empty seeds could be that some annual plants with high seed setting capacity was able to produce a lot of seed shells early in the season and, if the resources are provided, fill them later in the season. Each seed may not represent very much energy for annual plants with high seed setting capacity. This hypothesis is supported by findings of Ogunremi (1986) who found that the percent of empty seeds in Helianthus annua was highest at early harvest time. The phenomenon with intact but empty seeds has been observed but not explained in some other dicotyledonous plant species (Ebadi et al. 1996, M. Philipp pers. com.), but to the authors knowledge the percent of empty seeds has never been recorded as high as in this study, where 90 % of the Chenopodium album seeds were empty. More knowledge is necessary to understand the biological mechanisms behind these results.
2.3.3.3 Species composition
Seed from four weed species made up 46 % of the number of weed seeds in the seed rain: Atriplex patula/Chenopodium album, Stellaria media, Aethusa cynapium and Polygonum aviculare. Leguizamon and Roberts (1982) investigated the topsoil of fields without crops in England and found four species accounting for 87 % of the seeds. This difference could be explained by the different calculations of means: Geometric means are used in this study, decreasing the influence of extremely high values, whereas Leguizamon and Roberts (1982) used arithmetic means. Using the arithmetic mean, the four species with most seeds in this study would account for 72 % of the total amount of seeds.
The species composition of generative plants was different from the species composition in the seed rain. Species like Stellaria media and Chenopodium album (very frequent in the seed rain) had a very high seed set per plant compared to species like Viola arvensis/tricolor ssp. tricolor and Aethusa cynapium (frequent in the vegetation). This is in accordance with previous findings of seed setting capacity of those species (Korsmo 1926) and can explain the differences in species composition between the vegetation and the seed rain. Also the time a weed plant requires from germination to seed set may varies between species, which influence the amount of seeds produced at harvest time.
Seeds from three weed species made up nearly 50 % of the wild seed biomass: Bilderdykia convolvulus, Stellaria media and Atriplex patula/Chenopodium album. Seeds from these species are known as food items for birds (Christensen et al. 1996). It is worth noting that more than 90 % of the total seed biomass on stubble fields consisted of spilt grains. Therefore, grains are the main sources of food available for birds foraging on stubble fields in autumn. In addition, many birds prefer eating grains rather than weed seeds (Christensen et al. 1996, Berthelsen et al. 1997).
Comparing species in seed rain and vegetation
This study showed a good qualitative accordance between the species found in the vegetation in July and in the seed rain in September. The lower total richness found in the seed rain compared to the vegetation has many explanations: 1) Some of the seeds can not be identified to species but only to genus, therefore the number of species present is possible higher than indicated (Table 2.9). 2) Some of the species found in the vegetation do not reproduce by seeds or have not reached the reproductive age (e.g. Equisetum sp., Sambucus nigra or Salix sp.). 3) Vegetation was studied on 3.6 m2 per plot, whereas the seed rain was just collected from 0.72 m2 per plot. The species-area relationship implies that more species would be found in the vegetation than in the seed rain samples. This is supported by the fact that most of the generative species had a scattered distribution and were only found few times in the vegetation study. 4) It is possible that one or two species in the seed rain have been overlooked (e.g. seeds from Kickxia elatine, which looks like a placenta from Anagallis arvensis). 5) Moreover, seeds less than 0.5 mm were excluded from the identification (e.g. Epilobium montanum or Juncus bufonius).
2.3.3.4 Evaluation of methods
The methods used in this study were suitable to reveal the qualitative and quantitative characteristics of the seed rain on stubble fields with high efficiency. The methods were also suitable to determine low seed densities over large areas. However, the methods had some disadvantages.
Random errors
The weight of a sample had a positive significant effect on the number of spilt grain in the sample. This suggests that the sampling method had an impact on the results. The weight of a sample depends among other things on the soil structure, the amounts of straw on the fields and the humidity of the soil at the time of suction. These factors varied a lot from field to field but they did not vary much between plots within a field.
Systematic errors
The estimate of the size of the seed rain was certainly an underestimation, because not all seeds have been sampled by the C-vac method. Firstly, numerous seeds might have germinated after rainfall. Genera like Lamium, Veronica and Viola spread seeds many weeks before harvest of the crop, and some of these seeds might have entered the soil seed bank as a result of earthworms or rainwash (Hurka & Haase 1982). Secondly, some species like Aethusa cynapium set seeds several weeks after crop harvest and the seeds are therefore not shed at the time of sampling. Finally, not all seeds lying on the soil surface will end up in the C-vac, since the effectiveness of the sampling by suction lies around 80 % (Jensen unpublished data ) and varies from species to species. Another, systematic error happens during the identification and counting under a magnifier on a white background, where it is common to miss small and light seeds (Gross 1990). This might also had happened in this study.
All these random and systematic errors implies, that the results found in this study should not be considered an exact measure of the seed rain on stubble fields after harvest. However, the results reveal the proportional distribution of seeds between dosages.
2.4 Summary of dosage effects
In Table 2.12 the dosage effects on all dependent vegetation and seed rain variables are summarised.
Table 2.12.
Summary of dosage effects on the weed vegetation and the seed rain. Percentage increases
(+) and decreases (-) in response variable at reduced dosages compared to estimated mean
values at normal dosage. Significance is indicated as follows: +: 0.05£
p<0.10, *: 0.01£ p<0.05, **: 0.001£
p<0.01 and ***: p<0.001.
Dosage |
Positively correlated with |
||||||
Response variable |
Crop |
Normal |
Half |
Quarter |
|||
Weed density |
Spring barley |
66 plants/m2 |
+30 % |
+47 % |
* |
||
Winter wheat |
18 plants/m2 |
+ 23 % |
+ 85 % |
* |
|||
Sugar beets |
15 plants/m2 |
+127 % |
*** |
191 % |
*** |
||
Species richness |
Spring barley |
14 species/plot |
+ 16 % |
** |
+ 33 % |
*** |
Weed density |
Winter wheat |
8 species/plot |
+ 34 % |
+ |
+ 37 % |
+ |
Weed density |
|
Sugar beets |
19 species/plot |
+ 3.5 % |
+ 18 % |
Weed density |
|||
Proportion of flowering plants |
Spring barley |
42 % |
+ 16 % |
+ 31 % |
|||
Winter wheat |
32 % |
+ 26 % |
+ 38 % |
||||
Sugar beets |
48 % |
- 16 % |
- 20 % |
||||
Proportion of flowering species |
Spring barley |
64 % |
+ 14 % |
+ |
+ 14 % |
+ |
Proportion of flowering plants |
Winter wheat |
47 % |
+ 12 % |
+ 56 % |
** |
Proportion of flowering plants |
||
Sugar beets |
61 % |
+ 6.9 % |
+ 13 % |
+ |
Proportion of flowering plants |
||
Density of weed seeds |
Cereals |
142 seeds/m2 |
+ 42 % |
+ 63 % |
Density of generative weed plants |
||
Density of spilt grains |
Cereals |
45 grains/m2 |
+ 83 % |
* |
+ 82 % |
** |
|
Biomass of weed seeds |
Cereals |
0.15 g/m2 |
+ 21 % |
+ 35 % |
Density of generative weed plants |
||
Biomass of spilt grains |
Cereals |
1.3 g/m2 |
+ 83 % |
+ |
+ 93 % |
* |
|
Species richness in seed rain |
Cereals |
11 species/plot |
- 10 % |
-0.4 % |
Number of generative species |
||
Proportion of damaged weed seeds |
Cereals |
27 % |
- 0.9 % |
- 36 % |
+ |
||
Proportion of damaged spilt grains |
Cereals |
24 % |
- 5 % |
- 22 % |
|||
This large-scale field study has illustrated that reduced pesticide dosage affects the weed vegetation in many ways and with profound impacts. A reduction in dosage, from normal to quarter dosage is followed by a considerably higher species richness and a higher density of weed plants. Furthermore, species have a higher probability to flower and set seeds at quarter dosages, so the fitness of surviving species is higher than at normal dosage. All these effects of reduced pesticide dosages on the vegetation may affect the fauna by improving the living conditions at low dosages.