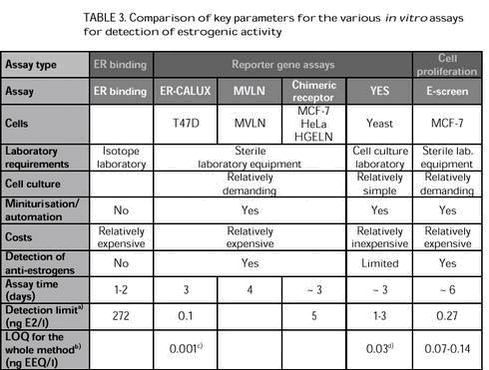
Evaluation of in vitro assays for determination of estrogenic activity in the environment6 Discussion and recommendationsNumerous substances present in the aquatic environment have been demonstrated to possess estrogenic activity. Chemical analysis of all compounds with potential estrogenic activity would be very costly and unknown estrogenic compounds, including metabolites, may still be present in environmental matrices. In vitro assays offer an integrated measure of the estrogenic potencies of environmental matrices without knowing all relevant compounds beforehand. In vitro assays can be utilized to examine considerable numbers of samples for their estrogenic activity. The sample preparation procedure often is the most tedious and time consuming step in monitoring using in vitro assays. However, sample preparation may be considered the most crucial part of the assessment process. Extensive environmental surveys involve the analysis of a large number of samples that cannot be immediately analyzed. However, extraction should be carried out as quickly as possible to avoid addition of chemical preservatives. It is important to note that when working with extracts of environmental matrices there may be loss of compounds during the extraction process. (Xeno)estrogens can be extracted by both solid-phase extraction and liquid-liquid extraction. Liquid-liquid extraction has been shown to be more efficient than solid-phase extraction. This may be due to the required filtration step preceding solid-phase extraction, which may result in loss of (xeno)estrogens adsorbed to suspended particulate matter. However, the conventional liquid-liquid extraction often requires considerable amounts of toxic organic solvents, and time-consuming procedures. Thus, solid-phase extraction could be preferred over liquid-liquid extraction. A large number of samples require a high sample throughput, which can be achieved by using a vacuum manifold system for solid-phase extraction cartridges. The filtration and extraction procedures for environmental samples should be properly validated and optimised before the onset of extensive monitoring studies. By using the appropriate extraction method it should be possible to assess estrogenic activity in different environmental matrices, such as wastewater, surface water, drain water and liquid manure slurry. A wide variety of in vitro assays has been developed to measure the estrogenic activity of single compounds or complex environmental samples. Each assay measures different end points at different levels of biological complexity of estrogen action (i.e., receptor binding, cell proliferation, and expression of a reporter gene). No single in vitro assay can be regarded as ideal for assessing the estrogenic activity of wastewater and surface water. They all have their advantages and limitations. A comparison of some of the key parameters for the various in vitro assays is shown in Table 3. Although they are fast, ER binding assays are significantly less sensitive than the other in vitro assays. In addition, binding assays are not easily amenable to automation, thereby limiting their utility as a screening tool. Furthermore, ER binding assays require specialised laboratory facilities because of the radioactive substances. Finally, the binding of a substance to the ER is only indicative that it may act as a xenoestrogen and some studies suggest, that ER binding may be a poor predictor of more complex in vitro and in vivo responses (Villeneuve et al., 1998). a) Detection limit not including concentration factors (see section 5.3.1). Although cell cultures involve disadvantages associated with maintaining the cell line and avoiding contamination, their use in test assays offers significant advantages regarding sensitivity. Both reporter gene assays and the E-screen have been successfully applied to assess estrogenic activity in surface water and wastewater in numerous countries. However, the long assay time (~6 days) for the E-screen is considered impractical for monitoring studies. In addition, a positive response cannot be attributed strictly to estrogen receptor agonists, since a variety of non-estrogenic substances has been found to influence the proliferation of MCF-7 cells. Furthermore, the E-screen and the mammalian-based reporter gene assays have the major drawback, compared to the yeast-based YES assay, that mammalian cells are more difficult and expensive to cultivate, and are more susceptible to cytotoxic effects. The advantages of in vitro assays over in vivo assays include lower costs and time consumption as well as sparing of experimental animals. However, in vitro assays do not always reliably predict the results of in vivo assays and should not be used alone for full assessment of potential estrogenic hazards in the aquatic system. In vitro the exposure of the cells is very direct without interaction with environmental factors influencing bioavailability and accumulation and without the toxicokinetics occurring in vivo. In vitro assays usually possess minimal metabolic capabilities. As a result, extrapolation from in vitro to in vivo systems can lead to false negatives for compounds that are bioactivated, and false positives for compounds that elicit an estrogenic response in vitro but are inactive in vivo as a result of rapid metabolic clearance. In addition, estrogenic effects in vivo include processes beyond the early simple events measured in vitro, and may involve complex interactions between different hormone systems, target tissues, and feedback loops. Furthermore, it should be kept in mind that there are estrogenic effects that are based on mechanisms different from receptor binding, e.g. interferences with hormone synthesis and metabolism. |