|
Mapping and release of chemical substances from products made of chloroprene 4 Screening analyses4.1 IntroductionBy experience the most suitable method for analysis of the liberation of volatile chemical substances from rubber is by headspace GC/MS methods (Rubber Fume). By this technique one gets soon a survey over the volatile or decomposition products, which might be liberated from the rubber. To detect less volatile chemical substances, e.g. anti-ageing agents and plasticisers an extraction followed by GC/MS analysis in combination with thin-layer chromatography is considered the most suitable method of analysis. The thin layer based method has the advantage that one by use of different spraying reagents can obtain different colours of the spots. This can give supplementary information regarding substance classes. Screening for heavy metals is most convenient carried out by X-ray analysis directly on the product. In the same analysis information is obtained regarding other chemical elements e.g. chlorine, sulfur, calcium, magnesium and alumina which might be present in the rubber recipy or as is the case chlorine in the chloroprene raw polymer. The chloroprene products purchased underwent screening analyses by x-ray analysing them for inorganic elements, including heavy metals, if any, thin layer chromatography for detection of anti-aging agents, a GC/MS analysis using headspace technique to detect volatile organic compounds, and a GC/MS analysis of a rubber extract in dichloromethane/isopropanol 90/10 vol.% in order also to determine the more slightly volatile compounds in the form of plasticisers. The analysis programme was stopped for one of the products that appeared not to be made of chloroprene, i.e. no. 3 dive gloves. The other pair of gloves, no. 7, that later appeared not to contain any chloroprene was included in the entire analysis programme. Having found that the gloves did not contain any chloroprene, the Danish Technological Institute contacted the importer and dealer of the product but they could not explain why the product did not contain any chloroprene. The product is marketed as titanium neoprene gloves. The supplier in England could not give a satisfactory explanation either. According to the importer, the manufacturer of the rubber-fabric composite is situated in the Far East. He did not believe that it would be possible to obtain any further details from the manufactor. 4.2 Description of analysis methods and test preparationThe analysis methods used in the screening phase are described briefly in the following. 4.2.1 X-ray analysisThe analysis was performed using a wavelength dispersive Phillips PV 2400 instrument. The analysis was performed directly on the cellular rubber without pretreatment. For some samples, an additional analysis of the rubber, as well as of the fabric and rubber, was performed. This involved samples containing so small amounts of chlorine that they could not be chloroprene products (despite the fact that they had been marketed as such). 4.2.2 Gas chromatography with mass spectrometric detection4.2.2.1 Headspace analysesApprox. ¼g of each rubber sample was cut into smaller pieces and placed in a 10ml vial with PTFE septum. The sample was heated for two hours at 100°C. An air sample was then taken using a gas-tight syringe and analysed by means of GC-MS. The content of volatile emission products was determined quantitively/semi-quantitively against selected compound standards heated in Pyrex bottles for evaporation and analysed in the same way as the rubber samples. The compounds used were carbon disulphide, diethylamine, toluene, aniline, phenol, DMF and 2-methoxyethanol. Equipment and parameters used:
4.2.2.2 Other GC/MS analysesThe samples were extracted using dichloromethane/isopropanol 90:10 vol.%.
4.2.3 Thin-layer chromatographic screening for anti-aging agents2g of rubber were weighed. The sample was placed in a 100 ml bulb and extracted using 50ml dichloromethane for an hour in a shaking bath. The solvent/extract was removed by decanting. An additional 10ml of dichloromethane was added and briefly shaken. Removal by decanting was then carried out again. The dichloromethane extract was evaporated using a rotary evaporator at max. 30°C to approx. 1ml. The concentrated extract was then transferred to a small test tube with a closely fitting cap. It was then rinsed with dichloromethane to achieve a total volume of 2ml. The thin layer chromatographic screening to identify the presence of anti-aging agents was carried out according to the principles of ISO 4645 (1995) ”Rubber and rubber products - Guide to the identification of antidegradants – Thin layer chromatographic methods”. Two different elution liquids were used. One of them (I) corresponds to method A in the standard: n-heptane:ethylacetate 90:10 (volume percentage). The other (II) elution liquid consisted of a toluene:acetone:ammonium hydroxide solution (item 4.3.13 of the standard) 100:20:0.2 (volume percentage). As for the latter method, it is possible to screen to identify the presence of ETU which is used, as already mentioned, as an accelerator in chloroprene recipes according to literature. The thin layer plates used for the analysis were Merck (article 1.11798) 20 x 20cm Silica gel 60 F 254 with concentration zone. Five micro litres were added of the solutions and standards, the production of which is described below. After eluation, the eluation liquid evaporated in a fume cupboard before visual assessment. The plates were first investigated under UV light and then after immersion in a chromatograpic chamber with iodine crystals. The results of the iodises plates were documented by photos. The reference substances used for the thin layer chromatographic screening are indicated in table 4.1 Table 4.1 References used for the TLC screening
The reference substances A, B, C, D, E and F were dissolved in dichloromethane as 1% solutions (0.1g in 10ml). Reference substance G (ETU) was dissolved in methanol (0.1g in 10ml) and thinned 1:4 in dichloromethane. Reference substance A (ODPA) was also produced in 2% and 5% strengths (0.2g and 0.5g in a 10ml measuring bulb) for the semi-quantitative assessment. 4.2.4 Estimated detection limitsThe detection limits for the various analysis techniques depend to a high degree of the compounds to be analysed for as well as interfering components and the method used. In table 4.2 typical detection limits based on experience are indicated for the analysis methods used. Table 4.2 Detection limits based on experience
As for the headspace analysis used for the screening for the more volatile constituents of the rubber, a semi-quantitative determination of the amount of selected individual components identified by the analysis at the exposure temperature of 100°C and a 2-hour period of exposure. The results are submitted and discussed under the result section. 4.3 Screening results4.3.1 X-ray analysisThe X-ray analysis gives a general view of which elements were included in the chloroprene rubber (from aluminium and upwards in terms of atom weight). The method is well-suited to screen for heavy metals in the rubber, such as nickel, but elements in environmentally problematic substances, such as tin in organotin compounds and arsen in arsen trioxide, would be identified by means of the analysis. Furthermore, it would be possible to get an indication of the content of chloroprene rubber in the product as well as other elements, such as sulphur, zinc, calcium, magnesium, aluminium and silicium, which are components typically found in the additives that form part of a chloroprene recipe. The results of the X-ray screening analysis are indicated in Table 4.3 and Table 4.4. Table 4.3 Results of X-ray analysis (weight percentage) Table 4.4 Results of x-ray analysis (weight percentage) It is remarkable that only two of the eight products purchased contained traces of the element chlorine. This concerned the two types of dive gloves nos. 3 and 7. This means that even though the products were purchased as neoprene products a different type of rubber was used to manufacture the gloves. As mentioned under section 3 "Samples purchased/acquired", the importer of one of these two types of gloves was not able to explain why the glove does not contain neoprene. It could have been foamed EPDM rubber since this type of rubber is extremely weather-resistant and widely used in the rubber industry. It should be pointed out that it was not demonstrated to be EPDM rubber. However, since both samples contained the basic elements, sulphur and zinc, which are substances characteristic of sulphur-vulcanised types of rubber, the presence of these elements in any case indicated that a type of rubber other than chloroprene was used in these gloves. The chlorine content for some of the other products was also remarkably low. This concerns the following products: dive socks (no. 5) and waders (no. 6). As both products contain the chemical elements sulphur and zinc, it is assumed that chloroprene is used in combination with another rubber. It should be mentioned that chloroprene is a medium prised rubber and money can be saved by use of cheaper rubbers like EPDM and SBR. It appears from the following considerations that are based on the fact that the sum formula of chloroprene is C4H5Cl if manufactured from polymerisation of 2-chlor-1.3-butadien alone. The content of chlorine in the polymer would then be 40.1% w/w, calculated on the basis of the sum formula. For sulphur-modified types, the chlorine content would be lower due to the integrated polysulphidic groups. The x-ray analysis of a chloroprene sample received from a Danish rubber factory showed a content of chlorine of 34% w/w. This could fit a sulphur-modified type even though the sulphur content was low. If we then look at the standard formulary mentioned in annex B, the content of chloroprene was 45.5% w/w. For a chloroprene type as the one used as reference (the sample from the rubber factory), this corresponded to a chlorine content in a dive suit of approx. 15.5%. For dive socks and waders (nos. 5 and 6, respectively), the x-ray analysis indicated a content of 1.9 and 1.8% w/w chlorine corresponding to a content of chloroprene raw rubber in the recipe of approx. 6-7 % w/w if based on the reference sample of 34% chlorine and the dive suit recipe. The chlorine content found in sample 2 (lower leg protector) and in sample 8 (dive suit) of 12 and 14% w/w, respectively, corresponded well in relation to that of a standard recipe. Sample 1 (knee bandage) and sample 4 (dive hood) have a chlorine content of 7.0 and 9.7% w/w, respectively, which still indicated that the content of chloroprene was fairly high and not atypical. With regard to the products with a low chlorine content Du Pont Dow Elastomers and Nordica Elastomers has been consulted. The result from the discussions with the latter (referred to by Du Pont Dow Elastomers) is that one was not able to give an example of a typical chlorine content in a chloroprene product as other rubber types could contain chlorine e.g. CSM ( chlorsulphonated polyethylene). However it was stressed that among divers Neoprene is equal to good quality and this might be the reason for the Neoprene labelling even in products without chlorine. Also the view was expressed that products with a low content of chlorine might be had a small addition of chloroprene to justify the Neoprene labelling. As to heavy metals, no lead was found in any of the products. Rather than zinc oxide, lead oxide is known to be used as an accelerator particularly for water-resistant types of chloroprene. However, smaller quantities of nickel were found in the 1, 2, 4 and 8 samples (knee bandage, lower leg protector, dive hood and dive suit). The levels ranged between 0.01% w/w and 0.06% w/w. It was characteristic that nickel was found in all the samples with a high content of chlorine (7 – 14 % w/w). There was no nickel in the reference raw chloroprene from the rubber factory. The source of contamination can be wearing parts from the mixer where the rubber compound is produced due to the intimate contact at this processing level between the chamber wall and the rotors which may have been made in a steel alloy with nickel. The nickel content in the rubber is so low that it is not likely that the limit value of 0.5 µg/cm² per week per cm² (Bekendtgørelse om nikkel, 2000)for nickel release would be exceeded in connection with contact attempts. Thus, based on the specific gravity of the foamed chloroprene of 0.11g/cm³, the content per cm² rubber of nickel was calculated to range between 1 - 6 µg for the total of the four samples where nickel was found. [5] In sample 3 (dive glove), the repeated analysis demonstrated an amount of 0.0067% w/w chromium on the surface of the fabric on the inside. As for the other gloves tested, chromium was also found, i.e. an amount of 0.0087% w/w for TI dive gloves and 0.026% w/w for Power neoprene gloves. This was localised to the fabric, not to the rubber. Apart from the magnesium content, there were no special remarks to be made regarding the other elements recognised since they might all originate from fillers added to the rubber, as mentioned initially. Magnesium forms part of a chloroprene rubber recipe in the form of oxide. This is to prevent untimely vulcanisation of the rubber as a consequence of the segregation of hydrogen chloride. The magnesium oxide thus functions as an acid catcher. It should be noted that the largest amount of magnesium is present in the products with a high content of chlorine, except for waders which tops the list with 1.4% w/w. The silicium content being high also for waders indicates that this product has been added magnesium silicate filler. Finally, it should be noted that no trace of the tin element was found in the products. So based on the x-ray screening, there was nothing to suggest that organotin compounds had been used as yeast and mould inhibitors in the products studied. 4.3.2 GC/MS headspace analysisThe headspace analysis by GC/MS was carried out at 100°C . This temperature is of cause much higher than the consumer exposure temperature, but is the most suitable test temperature to determine volatile chemical substances in the rubber. In all investigated samples a row of volatile chemical substances were revealed during the two-hour exposure time. Only one of the dive glove samples (no. 7) was tested because the Technological Institute left out glove sample no. 3 according to agreement with the National Agency of Environmental Protection since it contained chlorine only at trace level. The chlorine content in diving glove (sample no. 7) was also quite low, however the analysis had been performed when the above agreement was made. In Table 4.5 Results from GC/MS-headspace screening, we have tried to list in which samples the different substances appear in the volatile emission and in which groups of substances. Table 4.5 Results from GC/MS-headspace screening
Minor volatile hydrocarbon emission from all the samples was also seen. The volatile emission was most significant from sample 2 (lower leg protectors) and sample 4 (dive hood). The volatile emission from samples 1 (knee protector) and 2 (lower leg protector), and from samples 6 (waders) and 8 (dive suit) consisted primarily of hydrocarbons with 15-20 carbon atoms (sample 6, though, 12-20 carbon atoms); however, the samples differed to some extent as to the precise composition of the volatile hydrocarbon emission. The volatile emission from sample 4 (dive hood) and sample 5 (dive socks) was different and consisted of two fractions. One fraction was isomers of dodecyl benzene while the other fraction was isomers of butylated dodecyl benzene. The isomeric dodecyl benzenes seem primarily to be branched around the benzylic position. To supplement the ranking on a scale from 1-5 which was made on the basis of the number of counts per chromatographic peak, the volatile emission level was determined semi-quantitatively for a representative range of the substances detected in the screening analysis. The results appear from Table 4.6. Table 4.6 Volatile emission levels from rubber samples heated for 2 hours at 100°C [7]
Table 4.6 Volatile emission levels from rubber samples heated for 2 hours at 100°C [8]
The general volatile emission levels for other compounds are considered to be about 1µg/g (1ppm) or lower. However with regard to isophorone the level is about 3µg/g, glycols have a level around 3 – 6 µg/g and the level of toluen from waders (no.6) is 21µ g/g. The last result is without question much higher than for any other identified substance in the headspace. Otherwise, many types of compounds were found during the investigation of other types of rubber, primarily EPDM rubber. This concerns breakdown products from thiuram disulphides which are widely used in sulphur vulcanisation of rubber (but not chloroprene). Thiurams, however, are used to control the integration of sulphur in the production of sulphur-modified types of chloroprene. We know from literature (Rubber Fume, R. Badura) and well as from the Technological Institute's own studies that thermal breakdown of thiuram disulphides leads to the formation of carbonyl sulphide, carbon disulphide, secondary amines, ureas, thiourea substances, isothiocyanates and formamides. Similarly, it has to be expected that the isothiocyantes can be transformed into isocyanates when reacting with zinc oxide. Anti-aging agents can result in the formation of ketones. It is a fact that volatile emission of aniline can also occur from rubber vulkanisates. This may originate from anti-aging agents. The level of volatile emission does not exceed the level found by the Technological Institute for types of rubber other than chloroprene (primarily EPDM). 4.3.3 GC/MS analysis of extractsThe results of the GC/MS screening of the dichloromethane/isopropanol extracts from the chloroprene product samples studied are indicated below. In this case the GC/MS analysis has been performed directly on extract from the rubber products. Table 4.7 The results of the GC/MS screening of the dichloromethane/isopropanol extracts
The result of the GC/MS screening supported the detection of some of the breakdown products from accelerators and anti-aging agents which had already been identified by the headspace analysis, e.g. ethylisothiocyanate, dibutylformamide and BHT. The number of identified substance components, however, is significantly lower. The advantage of this method is that it provides us with additional information about the slightly more volatile components in the form of plasticisers and anti-aging agents. Thus, the presence of the DEHP phthalate plasticisers was also identified in the sample nos. 1 (knee bandage), no. 3 (dive gloves) and no. 7 (dive gloves). The amounts in 1 and 3 were at trace level whereas concentrations in no. 7 (dive gloves) were significantly above trace level. Dibutylphthalate was found in the sample nos. 1 (knee bandage), no. 3 (dive gloves) and no. 5 (dive socks). Sample 1 involved traces whereas the amounts in sample 3 (dive gloves) and no. 5 (dive socks) exceeded the trace level significantly. It was not possible to analyse the samples for chlorinated paraffines due to the content of plasticisers in the form of mineral oils. However, the very low content of chlorine in the x-ray screening indicated that chlorinated paraffines were not present in the products. Even in the few samples where the chlorine content approached the expected level for a typical chloroprene recipe, the content was not so high that the presence of chlorinated paraffines could be suspected. DOPA, which is an anti-aging agent, was found in all the samples, except in the dive glove sample no. 3. 4.3.4 TLC screeningsThe results of the TLC screenings are indicated in Table 4.8. This method was used to screen for content of anti-aging agents and ETU (ethylene thio urea). Table 4.8 Results of TLC screenings [9]
The thin layer chromatographic screening verified that anti-aging agents in the form of ODPA or bisdiphenylamine (references A and B) had been added. Based on the running of different concentrations of A, the concentration in the samples was estimated at approx. 0.5% w/w in the rubber. No ODPA/bisdiphenylamine was found in sample 3 (dive gloves) or in no. 5 (dive socks). Octylated diphenylamine was found in the GC/MS analysis but the amount was smaller than in the other samples. TLC screening will probably have to be performed for a more concentrated sample of no. 5 to obtain a positive screening result for this sample. A positive result was obtained for the presence of 6PPD in sample no. 3 (dive gloves). For sample no. 2 (lower leg protector), sample no. 7 (dive gloves) and sample no. 8 (dive suit) the IPPD result was positive. The concentration levels for these anti agening agents were significantly lower than for ODPA and hardly exceeds 0.1% w/w. ETU was not found in any of the samples during screening. It is known from literature that ETU is transformed during the vulcanisation process into its corresponding urea (without sulphur) (Röthenmeyer).
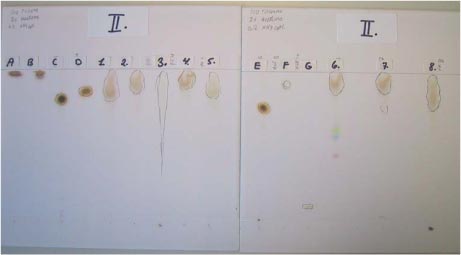
Photo 4.1 Example of TLC in eluation system II of reference substances and extracts of samples after development using iodine. Footnotes [5] Late in the project, the nickel salt of dimethyl dithiocarbamate or dibutyl dithiocarbamat was identified as being used as an efficient antiozonant (R. Kuschel, Rubber Handbook) in certain rubber recipes. In the "real life" diving experiment, the release of nickel was therefore measured based on this new knowledge. [6] The recognised organic compounds were detected only in the products where they had been given a mark ranging between 1 and 5. The numbers indicate the relative areas of the peaks in the chromatogram. The figure 1 indicates a high concentration in comparison with other peaks present. The figures 4 and 5 indicate that the concentration is rather low in the chromatogram. [7] The following remarks should be made to the volatile emission levels: The quantisising limit was established as the lower limit at which a "reasonable" quantitative value can be determined. Minus (-) means "not detected" 1): The content was determined quantitatively in relation to carbon disulfide. For carbonyl sulfide, this meant that the indicated values were probably maximum values wheras it was not directly possible to assess whether the values for ethylisothiocyanate were minimum or maximum values. 2): The content was determined quantitatively in relation to diethylamine. Since the response factors for a given type of compound are known, from experience, to increase as a function of the number of methylene groups (up to a certain limit), the level indicated must be considered the maximum value. The lower quantisising limit is due to the chromatography of dibutylamine being better than diethylamine. [8] The following remarks should be made to the volatile emission levels: The quantisising limit was established as the lower limit at which a "reasonable" quantitative value can be determined. Minus (-) means "not detected" 1): The content was determined quantitatively in relation to carbon disulfide. For carbonyl sulfide, this meant that the indicated values were probably maximum values wheras it was not directly possible to assess whether the values for ethylisothiocyanate were minimum or maximum values. 2): The content was determined quantitatively in relation to diethylamine. Since the response factors for a given type of compound are known, from experience, to increase as a function of the number of methylene groups (up to a certain limit), the level indicated must be considered the maximum value. The lower quantisising limit is due to the chromatography of dibutylamine being better than diethylamine. [9] The character "+" means "identified" and "–" means "not identified". A start (*) means that the TLC method cannot distinguish ODPA from bisdiphenylamine. When "+" is indicated in the ODPA column, it is because this is the substance component verified by GC/MS in the samples.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||