Effects of azole fungicides on the function of sex and thyroid hormones
1 Introduction
1.1 Background
Exposures to pesticides during early development may cause permanent damages to the nervous and reproduction systems (Nielsen et al., 2001). Therefore, children and fetuses (i.e. pregnant women) are particularly vulnerable towards pesticide exposure. This is due to the fact that the nervous and reproductive organ systems are not fully developed until late childhood and children have a higher dietary intake in relation to body weight than adults (Nielsen et al., 2001). Besides, children often consume relatively much fruit and juice, which can be polluted with pesticides. Very little is known about the potential health risks at low chronic exposures but even low concentrations of endocrine disrupting pesticides in the diet might be of concern due to potential combination effects (Birkhoj et al., 2004;Rajapakse et al., 2002;Silva et al., 2002).
Pesticides approved in Denmark have to be tested for adverse effects on reproduction and offspring by OECD guidelines TG 414 (Pre-natal Developmental Toxicity Study), 415 (One Generation Reproduction Toxicity Study), 416 (Two Generation Reproduction Toxicity Study), and 421 and 422 (Reproduction/Developmental Toxicity Screening Test) (http://www.oecd.org/home/). However, these test systems do not have an optimal design for revealing functional disruptions in the reproduction and nervous systems.
Recently we tested a range of commonly used pesticides for the ability to disrupt the endocrine system using a battery of in vitro tests (Andersen et al., 2002; Long et al., 2003). The pesticides were chosen due to their frequent use in Danish greenhouse horticultures (Andersen and Nielsen, 2001). Two thirds of the pesticides had endocrine disrupting properties and induced significant response in one or more of the in vitro tests. One of these pesticides was the imidazole fungicide prochloraz, which interacted in all the test systems. Prochloraz possessed anti-estrogenic and anti-androgenic effects, inhibited the activity of the estrogen synthesizing enzyme aromatase and activated the aryl hydrocarbon receptor (AhR). The AhR (or dioxin receptor) is an intracellular receptor that mediates the toxic responses of dioxin and related chemicals. Binding of a ligand to the receptor induce gene transcription of the CYP1A gene family and thereby the activity of some cytochrome P450 enzymes involved in the synthesis or metabolism of steroid hormones are increased (Rifkind, 2006).
In later studies, we found that prochloraz also induced anti-androgenic effects in rats in vivo (Vinggaard et al., 2002) and feminized male offspring in rats after perinatal exposure (Vinggaard et al., 2005a). The effects observed for prochloraz were comparable to effects reported for the fungicide vinclozolin (Gray, Jr. et al., 1994;Gray, Jr. et al., 1999a), which was banned because of its anti-androgenic effects. Furthermore, prochloraz decreased the concentration of thyroxin (T4) and thyroid stimulating hormone (TSH) in serum of exposed rats (Vinggaard et al., 2005a) indicating interference with the thyroid function.
In Denmark the use of fungicides has been increasing with a total sale of 1625 t in 2001, 1744 t in 2003, and 2046 t in 2005. (Miljøstyrelsen, 2004 ;Miljøstyrelsen, 2006). The azole fungicides are used in large amounts in the control of fungi in grain and to a lesser extent in flower, vegetable and fruit production. The total amount of azole fungicides (as active ingredients) sold in Denmark in 2005 was over 100 t. Epoxiconazole alone accounts for 47 t (approximately half of the azole fungicides used) and prochloraz, propiconazole, and tebuconazole, accounts for 2, 31, and 26 t, respectively (Miljøstyrelsen, 2006).
The azole fungicides are relatively lipophilic and are absorbed through the gastrointestinal tract (Kampmann et al., 1999). Therefore, the population can be exposed to azole fungicides through residues in the food. Residues of some azole fungicides are measured in fruit and vegetables but only few azoles are monitored in grain and grain products and for instance the most widely used azole fungicide in Denmark, epoxiconazole, is neither monitored in fruit, vegetables, grain nor grain products (Christensen et al., 2006). Assessment of the total dietary exposure to azole fungicides of the human population is therefore hampered. Although the maximal residue limit was not exceeded for the individual azole compounds included in the 2005 survey, prochloraz or tebuconazol was detected in several samples of fruits and vegetables produced in Denmark or imported (Christensen et al., 2006).
It is well known from the literature that several azole fungicides influence the activity of different cytochrome P450 enzymes and for instance inhibit the activity of aromatase (CYP19) which converts androgens to estrogens (Mason et al., 1987; Sanderson et al., 2002; Sanderson, 2006). Besides some in vitro studies on enzyme activities and our previous studies on prochloraz, only few other toxicological studies of these pesticides have been published despite of their massive use in agriculture and horticulture. In one study, exposure of pregnant rats to tebuconazole in doses around Lowest Observed Effect Level (LOEL) caused reduced weight of adult epididymis in male offspring and reduced adult uterus weight in female offspring. In addition, changes in behavior were observed (Moser et al., 2001).
Prochloraz, propiconazole, and tebuconazole were assessed by the Danish Environmental Protection Agency (EPA) in 1998, 1996, and 1996, respectively (Pesticidkontoret, 1996a; Pesticidkontoret, 1996b; Pesticidkontoret, 1998). The toxicological assessments were mainly based on the studies reported by the manufactor in order to get the product approved by the authorities. At that time, none of the pesticides were assessed to be toxic to the reproduction system. Both prochloraz and tebuconazole were reported to disturb the thyroid function but this was not considered to be of importance to human health. Prochloraz was re-assessed in 2004 and based on new studies and a review of the endocrine disrupting effects of the compound from The Danish Institute for Food and Veterinary Research, prochloraz was categorized as toxic to the reproductive system and labeled with R61: “May cause harm to the unborn child” (Pesticidkontoret, 2004). Epoxiconazole was assessed in 2003 (Pesticidkontoret, 2003) and, classified as carcinogenic and toxic to the reproductive system in category 3 and labeled with R40, R62, and R63.
So far the research in endocrine disrupting mechanisms has primarily focused on disruption of sex hormones and related receptors but other mechanisms are also involved (Sharpe, 2006). Interference with steroid hormone biosynthesis (Sanderson, 2006) or the thyroid system (Boas et al., 2006) are other important pathways of endocrine disruption.
The thyroid system is a central component of the endocrine system and thyroid hormones are essential for normal development and even small changes in thyroid homeostasis during critical periods of brain development may result in irreversible neurobiological damages and disruption of corresponding functions (Boas et al., 2006; Porterfield, 1994; Porterfield, 2000). The fetus itself, do not produce thyroid hormone during the first half of the pregnancy and is consequently depending on the mothers hormones. Chemicals that can interfere with the thyroid hormone level of the mother are therefore suspected to be able to cause neurological damages to the child (Boas et al., 2006; Porterfield, 1994). Ethylene thiourea, a metabolite of dithiocarbamate fungicides, is an example of a chemical that interferes with the thyroid system and causes thyroid hyperplasia and declined thyroid hormone levels (Lentza-Rizos, 1990). Different mechanisms of actions may be involved. The chemicals may bind directly to the thyroid hormone receptor (TR) or to the transport protein transthyretin or they may affect the release of TSH or the breakdown of thyroid hormones. For instance, the pesticides ioxynil, dicofol, and pentachlorphenol have been demonstrated to compete with triiodthyronine (T3) for the binding to transthyretin while they do not bind to the TR (Ishihara et al., 2003b; Ishihara et al., 2003a).
Our research so far on prochloraz, combined with the few other studies of toxic effects of azole fungicides, indicate that these compounds have the potential to react through several mechanisms resulting in effects on various organ systems (Vinggaard et al., 2005a). Our concern was that the endocrine disrupting properties demonstrated for prochloraz also applied to other azole fungicides, including triazole and other imidazole compounds. Hence, the aim of this study was to investigate if three other commonly used azole fungicides: epoxiconazole, propiconazole, and tebuconazole, possess comparable effects to those observed for prochloraz regarding interactions with the ER, AR and AhR in vitro as well as anti-androgenic and developmental effects in vivo. In addition, we wanted to investigate if prochloraz and the three other azole compounds interact with the TR in vitro.
Figure 1 illustrates the structure of the four azole fungicides. Prochloraz is an imidazole whereas the other three are triazoles. There is also a difference in the number of atomic chlorine attached to the phenyl rings and epoxiconazole differ from the others by containing atomic fluorine.
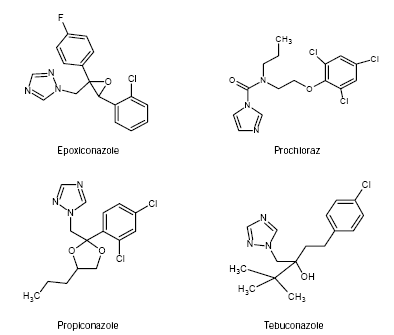
Figure 1 - Chemical structures of the four azole fungicides: epoxiconazole, prochloraz, propiconazole, and tebuconazole.
1.2 Project Objectives
The main objective of the project was to investigate the effects of three frequently used triazole fungicides on the endocrine system, including in vitro and in vivo examinations. Secondly, we wanted to compare the results with the imidazole fungicide, prochloraz, which previously has shown endocrine disrupting effects in a number of in vitro and in vivo studies.
1.3 Hypothesis and Strategy
The strategy of the project was to investigate:
1. Whether the triazole fungicides epoxiconazole, propiconazole, and tebuconazole possess similar properties as the imidazole fungicide prochloraz regarding interactions with the ER, AR, AhR, as well as effects on steroid hormone synthesis and the activity of the enzyme aromatase that converts testosterone to estrogen. The effects are investigated by established in vitro assays and the results are compared to the effects previously obtained for prochloraz. Differences in effects between the triazoles and prochloraz may indicate that the nature of the azole ring and/or the placement of the halogens in the phenyl rings, have an impact on the biological effect (Figure 1).
2. Whether prochloraz and the three triazoles interact with the TR. Tebuconazole and prochloraz have shown effects on the serum concentration of thyroid hormones in animal studies. The mechanism behind this effect is not known but one possibility is that the azole compounds may bind directly to the TR. Chemicals with the ability to react both with the ER, AR and AhR might be capable to also react with other receptors or binding sites as reported for several other environmental toxicants (Laws et al., 1995; Molina-Molina et al., 2006; Sanderson, 2006). We therefore wanted to investigate the molecular interaction of the four azole fungicides with the thyroid receptor. A T-screen assay was established for this purpose.
3. Whether the three triazoles induce anti-androgenic effects in adult male rats (Hershberger assay). If so the effects will be compared with the effects previously obtained for prochloraz. Concentrations of pituitary and thyroid hormones and changes in expression of androgen responsive genes will be included to increase the sensitivity of the assay. Changes in gene expression, in the rat ventral prostate, are a valuable marker of anti-androgen action. Many genes contain an androgen-responsive-element (ARE) in the promoter region and their expression is directly influenced by the amount of androgens or anti-androgens available (Nellemann et al., 2005).
4. Whether exposure to epoxiconazole or tebuconazole during pregnancy and lactation will cause effects on the offspring in rats. The fetus is the most sensitive stage of the organism towards endocrine disrupters. In an earlier study, feminization of male offspring was demonstrated after prochloraz exposure during pregnancy and lactation. The effects included feminization of male genitals, female-like areolas and feminized behavior. It is therefore highly relevant to investigate whether other azole fungicides induce similar endocrine disrupting effects during development.
Version 1.0 October 2007, © Danish Environmental Protection Agency