Effects of azole fungicides on the function of sex and thyroid hormones
2 Methods for testing endocrine disrupting effects of azole fungicides
- 2.1 In vitro testing
- 2.1.1 Estrogen/anti-estrogen testing – MCF-7 cells
- 2.1.2 Aromatase testing – MCF-7 cells
- 2.1.3 Cytotoxicity inMCF-7 cells
- 2.1.4 Androgen/anti-androgen testing – AR-assay
- 2.1.5 Ah receptor testing – CALUX assay
- 2.1.6 Steroid synthesis testing – H295R cells
- 2.1.7 Thyroid testing – GH3 thyroid assay (T-Screen)
- 2.2 In vivo testing
- 2.3 Statistical analyses
2.1 In vitro testing
The first step in investigating endocrine disrupting properties of the azole fungicides was to screen the four compounds: epoxiconazole, prochloraz, propiconazole, and tebuconazole in a battery of cell assays for well-known mechanisms of endocrine disruption. The assay systems included the ability to induce proliferation in a human breast cancer cell line (MCF-7 cells) (estrogenic/anti-estrogenic effects), agonistic and antagonistic effects on the AR in transfected Chinese Hamster Ovary (CHO) cells, effects on activity of the estrogen synthesizing enzyme aromatase in the MCF-7 cell proliferation assay using testosterone as substrate, dioxin like effects estimated as activation of the Ah receptor in the CALUX assay, effects on thyroid function, by looking at the thyroid dependent cell proliferation of a rat pituitary tumour cell line (GH3 cells) in the T-Screen assay and finally effects on steroidogenesis in human adrenocortical carcinoma cells (H295R cells).
2.1.1 Estrogen/anti-estrogen testing – MCF-7 cells
The azole fungicides were tested for estrogenic and anti-estrogenic effects using a MCF-7 cell proliferation assay. The assay is based on a human breast cancer cell line. The cells depend on estrogen for growth. Proliferation of the cells is therefore an indication of the presence of an estrogen like compound. The method is used on a regular basis in many research laboratories including the laboratory at Environmental Medicine, University of Southern Denmark. The MCF-7 cell proliferation assay is considered to be the most reliable in vitro assay. It is of more humane physiologic relevance than other established in vitro assays as it is based on a mammal cell line which has not been manipulated genetically making the regulation of the estrogenic response more naturally (Andersen et al., 1999).
In short, MCF-7 BUS cells were seeded in microtiter plates and after 24 h, dilutions (0.001-150 µM) of the azole fungicides were added for six days of incubation. The azole fungicides were tested with and without 10 pM 17β-estradiol (which induces half of the maximum proliferation response in the cells) to detect both antagonistic and agonistic effects. Cells were fixed and stained. The number of living cells was measured using an ELISA-reader. Each experiment included a standard curve of 17β-estradiol ranging from 1 pM to 10 nM and the maximum response was detected at 1 nM. The assay is very sensitive with a limit of detection of 0.5 pM 17β-estradiol and a quantification limit of 1 pM 17β-estradiol. Only results obtained with a standard curve slope within two times the standard deviation (estimated from a large number of independent experiments) was accepted. Furthermore, the mean control activity (based on minimum 3 wells) in each plate had to be within two times the standard deviation of controls to be included. This ensured a normal response from the cells and excluded increased background activity due to contamination. Each azole fungicide was tested in at least three independent experiments and in twenty-two different concentrations (0.001, 0.005, 0.01, 0.05, 0.1, 0.2, 0.39, 0.5, 0.78, 1, 1.56, 3.13, 5, 6.25, 10, 12.5, 30, 50, 75, 100, 125, 150 µM) in triplets. Results are presented as the Relative Proliferative Effect (RPE) in which data are normalized both to untreated control cells and the maximum response induced by 1 nM 17β-estradiol in each experiment:
RPE = [(PEtest sample-1)/(PEmax17β-estradiol-1)] x 100, where PE correspond to the response normalized in relation to control. The concentration-response relationships were plotted and fitted to the sigmoidal function: y=y0+a/[1+(x/x0)b]
2.1.2 Aromatase testing – MCF-7 cells
The MCF-7 cells express the enzyme aromatase naturally. The enzyme converts testosterone to estrogen and hence induce proliferation of the cells (Sadekova et al., 1994;Sonne-Hansen and Lykkesfeldt, 2005). By co-treating the cells with testosterone and fungicides an inhibition or stimulation of the enzyme activity can be registered (Almstrup et al., 2002). Previously, the assay has been used (using 1 µM testosterone which induces about 65% of the maximum response of 17β-estradiol) for testing effects on aromatase activity by the fungicide fenarimol (Andersen et al., 2006). The results obtained were in good agreement with results obtained using other aromatase assays. Prochloraz has been demonstrated to inhibit aromatase activity in different in vitro assays (Andersen et al., 2002;Vinggaard et al., 2000). By including prochloraz in this study it is possible to confirm the sensitivity of this assay compared to other assays. Since the same assay system is used for investigating estrogenicity and effects on aromatase it is possible to compare directly the concentrations inducing these effects.
Each azole fungicide was tested in three independent experiments at sixteen different concentrations (0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20, 25, 30, 40, 50, 75, 100 µM) in triplets. Results are presented as RPE.
2.1.3 Cytotoxicity inMCF-7 cells
Cytotoxicity of the fungicides was evaluated by using the Promega Cytotox 96 Non Radioactive Cytotoxicity assay. Briefly, the MCF-7 cell proliferation assay was set up as usual for testing of estrogenicity or aromatase activity but with identical plates in pairs as the only difference. This was done to be sure that the assay passed as expected at the same time as the cytotoxicity could be measured. On the sixth day of exposure half of the plates were stopped as normal and the other half continued in the cytotoxicity assay.
The cytotoxicity was assessed indirectly by measuring the stable cytosolic enzyme lactate dehydrogenase (LDH) released from damaged cells before and after lysis. The LDH activity was measured by an enzymatic assay where tetrazolium salt converts into a red formazan product and where the subsequent measured absorbance then corresponds to the number of lysed cells (Promega, 2001). The more cytotoxic a compound is the more LDH will be released during the six incubation days of the assay and the less will be released after lysis. The maximum release corresponds to the total release of LDH before and after lysis. The cytotoxicity was assessed by calculating the ratio:
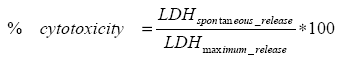
Data will not be shown, but indicated in the relevant figures.
2.1.4 Androgen/anti-androgen testing – AR-assay
Effects on AR activity were tested in a reporter gene assay (Vinggaard et al., 1999) with minor modifications. Chinese Hamster Ovary cells (CHO K1) were maintained in DMEM/F12 (Gibco, Paisley, UK) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B (Sigma-Aldrich, St.Louis, MO) and 10 % fetal bovine serum (FBS; BioWhitaker, Walkersville, MD). The cells were seeded in white 96-well microplates (PerkinElmer Life Sciences, Packard) at a density of 7,000 cells per well in DMEM/F12 containing 10 % charcoal-treated FBS (BioWhitaker) and incubated at 37°C in a humidified atmosphere of 5% CO2/air. After 24 h cells were transfected for 5 h with a total of 75 ng DNA per well consisting of the expression vector pSVAR0 and the MMTV-LUC reporter plasmid (both provided by Dr. Albert Brinkmann, Erasmus University, Rotterdam) in a ratio of 1:100 using 300 nl of the transfection reagent FuGene (Boehringer Mannheim, Germany). The ratio of DNA (μg) to Fugene (μl) was kept at 0.25. The fungicides were tested in twelve concentrations ranging from 0.025 to 50 μM, combined with 0.1 nM of the AR agonist R1881 (NEN, Boston, MA). The test solutions were prepared from 10 mM stock solutions in ethanol. After incubation for 20 h, the media was aspirated and the cells were lysed by adding 20 μl per well of a lysis buffer containing 25 mM trisphosphate, pH 7.8, 15 % glycerol, 1 % Triton X-100, 1 mM DTT and 8 mM MgCl2, followed by shaking at room temperature for 10 min. Luciferase activity was measured directly using a Lumistar Galaxy luminometer by automatically injection of 40 μl substrate containing 1 mM luciferin (Amersham Int., Buckinghamshire, U.K.) and 1 mM ATP (Boehringer Mannheim, Germany) in lysis buffer and the chemiluminiscense generated from each well was measured over a 1 sec interval.
Cytotoxicity experiments were performed as described above, for the cell-procedures and transfections, except that the pSVAR0 expression vector was replaced by the constitutively active AR expression vector pSVAR13 (a gift from Brinkmann), which lacks the ligand-binding domain of the receptor. The ratio between pSVAR13 and MMTV-LUC was 2:100.
2.1.5 Ah receptor testing – CALUX assay
The rat hepatoma H4IIE cells were stably transfected with the PAH/HAH-inducible luciferase expression vector pGudLuc1.1. This vector contains the firefly luciferase gene under PAH/HAH-inducible control of four murine DREs (dioxin responsive elements) inducing luciferase in a time- and dose-dependent manner (Garrison et al., 1996).
H4IIE cells were grown in α-MEM medium with 5% FBS at 37°C in 95% air and 5% CO2. Cells were seeded into sterile 96-well plates at 22.1 x 104 per ml and plates were incubated for 24 h prior to compound exposure, allowing cells to reach 90-100% confluence. Cells were exposed for 24 h to the azole fungicides (0, 0.2, 0.4, 0.8, 1.6, 3.1, 6.3, 12.5, 25, 50 mM) in α-MEM supplemented with 1% FBS. Following exposure, cells were washed twice with PBS (pH 7.4) and lysed in 20 µl lysis buffer. An aliquot of 10 µl supernatant was pipetted into a 96-well microtitre plate and luciferase activity was determined using a Lumistar Galaxy luminometer.
2.1.6 Steroid synthesis testing – H295R cells
The H295R cell line, which is derived from human adrenocortical carcinoma cells, produces a wide range of steroid hormones in measurable quantities, including testosterone, progesterone and estradiol. This property makes the cell line suitable as a screening assay to detect effects on steroidogenesis. One alternative to the H295R cell line is the mouse Leydig tumour cell line MLTC-1. It produces progesterone, but only small amounts of testosterone and estradiol, which makes it less suitable as a screening assay to detect chemicals affecting steroid synthesis.
H295R cells (ATCC, CRL-2128) established from a pluripotent human adrenocortical carcinoma cell line were grown in 24-well culture plates (Costar, Corning, NY, USA) at 37°C humidified atmosphere of 5% CO2/air. Each well contained 1 ml DMEM/F12 medium (GibcoBRL Life Tachnologies, Paisley, UK) supplemented with 2.0% Nu-serum (BD Sciences, Denmark), ITS+ premix (containing 6.25 mg/ml insulin, 6.25 µg/ml transferin, 6.25 μg/ml selenuim, 1.25 μg/ml BSA and 5.35 μg/ml linoic acid) and 100 U/ml penicillin, 100 mg/ml streptomycin and 250ng/ml amphotericin B (Fungizone®). The cells were plated at a density of 2x105 cells/well and allowed to settle for 24 h. Culture medium was removed and new medium containing fungicide dissolved in dimethyl sulfoxid (DMSO) was added to the cells in triplicates (prochloraz: 0, 0.01, 0.03, 0.1, 0.3, 1, and 3 mM, epoxiconazole, propiconazole, and tebuconazole: 0, 0.1, 0.3, 1, 3, 10 and 30 µM). Control wells contained the same amount of DMSO (0.1%) as exposed cells. After incubation for 48 h, the medium was removed and stored at -20°C until measured for testosterone, progesterone and estradiol levels. Samples of 800 µl were concentrated on IST Isolute® SPE columns (100mg, C18, 1ml) (Hengoed, UK), and the steroid hormones were measured using Delfia kits (PerkinElmer Life Sciences, Turku, Finland).
After exposure, the cells were incubated with resazurin solution to test for cytotoxicity. Medium from these wells (200µl) was transferred to black microplates (Costar, Corning, NY, USA) before fluorescence was measured. No cytotoxicity was observed for any of the concentrations used.
2.1.7 Thyroid testing – GH3 thyroid assay (T-Screen)
For the in vitro detection of agonistic and antagonistic properties of the azole fungicides towards the TR, the relatively new test method, called the T-screen, was used. The assay is based on the thyroid hormone dependent cell growth of a rat pituitary tumor cell line (GH3). This cell line expresses intracellular TR in very high amounts, and the assay can be used to study interference of compounds with thyroid hormone at the cellular level.
The GH3 cells were cultured at 37°C humidified atmosphere of 5% CO2/95% air in DMEM/F12 (Gibco, Paisley, UK) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B (Sigma-Aldrich, St.Louis, MO) and 10 % fetal calf serum (FCS)(Gibco, UK). Forty-eight hours prior to plating the cells into 96-well microplates (Costar, Fisher Scientific Biotech Line) for the experiment, standard culture medium was changed to test medium containing 10% (v/v) T3- and T4-depleted dextran-charcoal treated FCS (DC-FCS). After 48 h in DC-FCS, which was changed once after 24 h, the GH3 cells were released using a cellscraper and seeded in 96-well black, clear bottom microplates, 50 μl cell suspension and 50 μl test compound per well, at a density of 2500 cells/well. The azole fungicides were dissolved in DMSO, and all test solutions were prepared from 20 mM stock solutions, except the T3 stock solution, which was 10 mM. All four azole fungicides were tested in triplicates (epoxiconazole, prochloraz, propiconazole, and tebuconazole: 0, 0.01, 0.375, 1, 3, 10 and 30 μM) and were tested both in the absence and presence of 0.25 nM T3 (the reported T3-EC50 by (Gutleb et al., 2005), to test for agonistic and antagonistic potency. Control wells contained cells and test medium with the same amount of DMSO (0.1%) as the exposed cells. The plates were incubated for 96 h, and cell growth was analyzed using AlamarBlue™ (BioSource, California, USA). 10% (v/v) AlamarBlue was added to each well, and the plates were incubated 4 h at 37°C, protected from light. The experiment was terminated and the plates were analyzed by measuring fluorescence (ex. wavelength 560 nm/em. 590 nm) on a Wallac Victor 1420 multilabel counter (PerkinElmer life Sciences, Turku, Finland).
2.2 In vivo testing
The second step in the investigation of endocrine disrupting properties of azole fungicides was to include animal studies. First a short-term animal study was conducted to study whether the azole fungicides were anti-androgenic in young adult rats. The results obtained in this study were used to design the following study in which pregnant rats were exposed to the fungicides to investigate endocrine disrupting effects on fetuses and offspring. This later assay is presently considered the most sensitive, but also a very comprehensive animal study, for testing of endocrine disruptors.
2.2.1 Anti-androgenic testing – Hershberger test
Male Wistar rats were acquired from Taconic M & B, Eiby, Denmark. 6 intact male rats (42 days old at the dosing start) and 54 males, castrated at an age of 4 weeks, 14 days prior to study start, were used. All animals were delivered 1 week prior to study start and were upon arrival housed in Bayer Makrolon type 3118 cages (Type: 80-III-420-H-MAK, Techniplast), three per cage with Tapvai bedding. They were fed Syn 8.IT (a diet known to be free of phytoestrogens) and were provided with acidified tap water ad libitum. Animal rooms were maintained on a 12-hour light/dark cycle, a temperature of 22 ± 1 °C and a relative humidity of 55 ± 5 %. Rats were weighed and divided by randomization into treatment groups so that there were no statistically significant differences among group body weight means. During testing rats were weighed daily and visually inspected for health effects twice a day.
One group of intact animals and 9 groups of castrated male Wistar rats, were included in the study (n=6 per group). The intact rats and the one group of castrated rats served as negative controls and were given peanut oil only. The rats in the remaining eight groups were all dosed with testosterone propionate (0.5 mg/kg/day sc). One group was only administered testosterone propionate and served as control group to which all other groups were compared. The positive control group received flutamide (10 mg/kg bw/day orally). The last 6 groups received propiconazole or tebuconazole orally at doses of 50, 100, or 150 mg/kg bw/day.
All compounds were dissolved in peanut oil. Sterile oil (the Royal Veterinary Agriculture Pharmacy, Copenhagen, Denmark) was used for the testosterone propionate solution. All compounds were administered in a dosing volume of 2 ml/kg body weight and the dosing period was 7 days for all animals. The testosterone dose was always given a few minutes after the test compound and the last dosing was performed in the morning at the day of killing the animals. Body weights were recorded and animals were euthanized using CO2/O2 followed by exsanguinations. All the animals from each group underwent a thorough autopsy. The testis (for intact animals), both lobes of the ventral prostate, combined seminal vesicles and coagulating glands including fluids, levator ani/bulbocavernosus muscle (LABC), paired bulbourethral glands, pituitary, liver and paired kidneys were dissected and weighed. Organ weights were calculated relative to body weights. The ventral prostates were put in 0.5 ml RNAlater (Ambion) and stored at -20°C until gene expression analysis. Blood was collected by heart puncture in plain glass tubes and serum was prepared and stored at -80°C until measurement of hormones.
2.2.1.1 Hormone levels
Rat luteinizing hormone (LH), rat follicle stimulating hormone (FSH), and T4 levels were analyzed in serum using the technique of time-resolved fluorescence (Delfia, Wallac). LH and FSH were analyzed at Turku University, Finland. Rat FSH immunoreactivity was determined by a two-site immunofluorometric assay (IFMA) (van Casteren et al., 2000). The standard used was a NIDDK standard FSH-RP-2 obtained from the National Hormone and Pituitary Program, NIH, Rockville, MD.
Rat LH was measured using the time-resolved fluorimetric assay (IFMA, Delfia, Wallac OY, Turku, Finland) as described (Haavisto et al., 1993). The standard rLH RP-3 was kindly provided by NIDDK, NIH (Baltimore, MD).
2.2.1.2 Gene expression levels determined by real-time RT-PCR
The organs were homogenized and total RNA was isolated using RNeasy-mini kit and RNase-Free DNase set (Qiagen). cDNA was synthesized from 0.5 µg total RNA using the Omniscript Reverse Transcription kit (Qiagen) with T16 oligoes and a 18S rRNA primer. Samples were quantified on the 7900HT Fast Real-Time PCR System (Applied Biosystems) by standard TaqMan technology. Expression levels of the following genes were quantified, in the ventral prostate: The androgen-responsive genes prostate binding protein C3 (PBP C3), ornithine decarboxylase (ODC), testosterone-repressed prostate message 2 (TRPM-2), complement component 3 (Compl. C3), the androgen receptor (AR) and insulin-like growth factor 1 (IGF-1). These genes were chosen because they have previously been shown to be androgen-regulated. PBP C3 is a tissue-specific protein and the principal secretory protein in rat prostatic fluid (see references in (Nellemann et al., 2001)). ODC is necessary for cell growth and differentiation as an important enzyme in the synthesis of polyamines. ODC catalyzes the conversion of ornithine to putrescine, which is the first and rate-limiting step in polyamine biosynthesis. TRPM-2 also called clusterin, is not normally expressed in the rat ventral prostate, but is induced by castration (Leger et al., 1987). TRPM-2 is expressed at high levels in dying cells, and data suggest that TRPM-2 acts as a survival factor from apoptosis (Ho et al., 1998;Ogawa et al., 2005;Viard et al., 1999). Compl. C3 is involved in the innate immune response and together with PBP C3, ODC and TRPM-2, this gene has been selected, because the expression of these four genes in ventral prostate, previously have been found to be valuable for investigating anti-androgenic effects in the Hersberger assay (Nellemann et al., 2001;Nellemann et al., 2005). For each sample, 2 µl cDNA (1.75 ng/µl) was amplified under universal thermal cycling parameters (Applied Biosystems) using TaqMan Fast Universal PCR Master Mix (Applied Biosystems) in a total reaction volume of 10 µl. Three separate amplifications were performed for each gene and when intra-assay variation was above 10% additional amplifications were performed. All genes were quantified from standard curves, and expression levels of each target gene were normalized to the expression level of the housekeeping gene 18S ribosomal RNA (18S rRNA). Table 1 illustrates the sequences of the primers used.
Table 1 - Sequences of the primers used for quantitative real-time PCR.
18S rRNA
5’-FAM-ACC GGC GCA AGA CGA ACC AGA G-TAMRA-3’
Forward, 5’-GCC GCT AGA GGT GAA ATT CTT G-3’
Reverse, 5’-GAA AAC ATT CTT GGC AAA TGC TT-3’
PBP C3
5’-FAM-TCA TCT AGA ATA CTG CAG CCA GAA CCA CTG G-TAMRA-3’
Forward, 5’-CCA TCC CCA TTT GCT GCT AT-3’
Reverse, 5’-AGT CAC AGT TGA GTT AAT TGT ACC TCT AAT AAC-3’
ODC
5’-FAM-ACT CAC TGC TGT AAC ACA CAG CCT GTG CA-TAMRA-3’
Forward, 5’-AAT GTG TGC AAG TAT CCC TTA CAG AA-3’
Reverse, 5’-CAC AGC TTT GTA TCA TCC ACA TCT C-3’
TRPM-2
5’-FAM-AGT TTC TGA ACC AGA GCT CAC CCT TCT ACT TCT G-TAMRA-3’
Forward, 5’-CTG GTT GGT CGC CAG CTA GA-3’
Reverse, 5’-ATG CGG TCC CCG TTC AT-3’
Compl.C3
5’-FAM-CGT AGT CCA CTC CAG GCT CAC AAG-TAMRA-3’
Forward, 5’-CAG CCT GAA TGA ACG ACT AGA CA-3’
Reverse, 5’-AAA ATC ATC CGA CAG CTC TAT CG-3’
IGF-1
5’-FAM-CAA CAC TCA TCC ACA ATG CCC GTC T-TAMRA-3’
Forward, 5’-GAC CAA GGG GCT TTT ACT TC-3’
Reverse, 5’- GCA GCG GAC ACA GTA CAT CT-3’
AR
5’-FAM-TCG CGA TTC TGG TAT GCT GCT GC-TAMRA-3’
Forward, 5’- GAC ACT TGA GAT CCC GTC CT-3’
Reverse, 5’- GAG CGA GCG GAA AGT TGT AG-3’
2.2.2 Developmental effects on offspring after perinatal exposure
To test for developmental effects, pregnant dams were dosed with epoxiconazole or tebuconazole and the fetuses and offspring were examined for effects on sexual differentiation. Important biological endpoints were: gestational length, survival, anogenital distance (AGD) in fetuses and offspring, retention of nipples in male pups, decreased weight of accessory sex tissues, semen quality, morphological effects on the external genitalia (hypospadia and cryptorchidism), hormone levels, and gene expression. Figure 2 is a schematic overview of the study design.
2.2.2.1 Animals and exposure
In short, 112 pregnant Wistar rats (HanTac: WH, Taconic M& B, Ejby, Denmark) were received on gestational day (GD) 3. The day after arrival, the animals were weighed and assigned to treatment groups (1-5) by stratified randomization to assure equivalent body weight means across groups prior to dosing. The rats were gavaged peanut oil (n=24), with 15 or 50 mg/kg bw/day epoxiconazole (n=24/24), or 50 or 100 mg/kg bw/day tebuconazole (n=20/20) respectively, from GD 7 to postnatal day (PND) 16. Animals were divided into two sets, such that 56 animals representing all 5 groups were dosed one week prior to the next 56 animals. In the 5 groups 19, 19, 18, 19 and 21 rats, respectively, were pregnant.
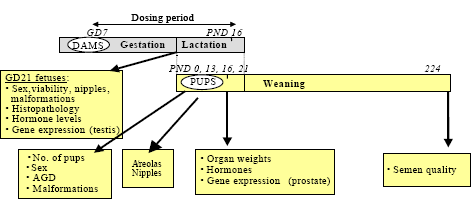
Figure 2 - Schematic overview of the design of the developmental toxicity study. Pregnant dams were dosed GD7 to PND 16. At GD 21 some of the dams were chosen for caesarian section and the fetuses were investigated. Some of the dams were allowed to give birth (PND 0). At PND 16 some of the offspring were killed and tissue was taken for hormone- and gene expression analysis as well as pathology. Some of the offspring continued in the study for behavior tests (not included in this report) and semen quality tests (PND 224).
GD: gestational day; PND: postnatal day; AGD: anogenital distance.
2.2.2.2 Health status of dams and delivery
The rats were observed twice daily for signs of toxicity. Body weight was registered daily from GD 7 to PND 16. The maternal weight gain from GD 7 to GD 21, GD 7 to PND 1, and PND 1 to PND 13 was calculated from the data. The first measure is based on the weights of the dams including the weight of the fetuses and may therefore, if affected, reflect an effect on the maternal animal and/or the fetuses. In contrast, the maternal weight gain from GD 7 to PND 1 as well as the maternal body weight on PND 1 provides measures of the dam body weight only. From GD 21 the rats were inspected twice a day to register time of birth. After delivery body weights of dams and pups were recorded. Number of pups, sex ratio and anomalies were registered. The day of delivery is PND 0.
2.2.2.3 Caesarian sections GD 21
At GD 21 the following numbers of dams were originally selected for Caesaeian section: 6 controls; 7 dosed with 50 mg/kg bw/day and 8 dosed with 100 mg/kg bw/day tebuconazole; 9 dosed with 15 mg/kg and 14 dosed with 50 mg/kg epoxiconazole. However, because of problems with parturition in the highest dose groups, caesarian section was additionally performed on 2 dams in the group receiving 100 mg tebuconazole/kg bw/day and on 4 dams in the group receiving 50 mg epoxiconazole/kg bw/day group. The dams were weighed and decapitated after CO2/O2 anesthesia. Uterus was taken out, and the number of live fetuses, re-absorptions, implantations and any anomalies was registered. The remaining dams (13, 12, 8, 10 and 3 dams from the control, the 50 mg/kg and the 100 mg/kg tebuconazole, the 15 mg/kg and the 50 mg/kg epoxiconazole group, respectively) were allowed to give birth.
2.2.2.4 Section of pups PND 16
At PND 16 all mothers and all pups except 72 pups, were killed and tissue was taken for hormone-, gene expression-, and pathology analysis. The 72 remaining animals continued in the study for behavioral tests (not included in this report) and semen quality analysis (34 male pups).
2.2.2.5 Histology and Immunohistochemistry GD 21
In fetuses at GD 21 one testis from 1 to 3 males per litter were placed in Bouin’s fixative, embedded in paraffin and one section per male was used for histopathology (haematoxylin and eosin stain) or immunohistochemistry. Immunohistochemistry was performed on one section per testis. Following microwave pretreatment for 2x5 min in either citrate or TEG buffer, sections were blocked for endogenous peroxidase activity in 3% H2O2 in phosphate buffered saline (PBS), and blocked in 1% bovine serum albumin in PBS. Sections were incubated over night at 4°C with the following rabbit polyclonal antibodies: Steroidogenic acute regulatory protein (StAR) antibody (PA1-560, Affinity Bioreagents, Golden, CO, 1:1000), cytochrome P450-side-chain cleavage (P450scc) antibody (AB 1244, Chemicon, Temecula, CA, 1:50,000), peripheral benzodiarepine receptor (PBR) antibody (SantaCruz, CA, 1:200), or 17β-hydroxysteroid dehydrogenase (17b-HSD) type 10 (Kem-En-Tec). Sections were then incubated for 30 min with secondary antibody (Anti-rabbit EnVision+, DAKO, Glostrup, Denmark), stained in diaminobenzidine (DAB+ Substrate Chromogen System, DAKO, Glostrup, Denmark) or 3-amino-9-ethylcarbazole (AEC, Labvision, CA), and counterstained in Meyer’s hematoxylin. Negative controls were fetal control testis, incubated with blocking serum instead of primary antibody.
2.2.2.6 Hormone analysis GD 21 and PND 16
Testosterone, progesterone, 17α–hydroxy-progesterone, estradiol and thyroid hormones were analyzed in rat plasma or testes at GD21 and/or PND 16. Steroid hormones were extracted from the serum on IST Isolute C18 SPE columns (200mg/3ml). The serum samples were diluted with purified water 1:1 and applied to columns, preconditioned and rinsed with methanol (MeOH) and water, respectively. Interfering substances were eluted with 2x2 ml MeOH:water (20:80 v/v) and steroids were eluted with 2x2.4 ml MeOH. The solvent in these fractions was evaporated and samples were resuspended in 100 μl Diluent 1 (PerkinElmer, Turku, Finland) and the steroids together with the thyroid hormones triiodothyronine (T3) and thyroxine (T4) were analyzed using a Delfia time-resolved fluorescence kit (PerkinElmer, Turku, Finland) and measured by use of a Wallac Victor 1420 multilabel counter (PerkinElmer life Sciences, Turku, Finland).
Steroid hormones were analyzed in testis and estradiol in ovaries after extraction with diethyl ether. Decapsulated testes or ovaries were placed in vials containing 100 or 500 μl water and 0.5 or 2.5 ml diethyl ether, respectively. The tissue was homogenized and the vials were placed in a tub consisting of dry ice and acetone until the water-fraction was frozen. The ether-fraction was transferred to a clean vial, the procedure was repeated, and the two extracts were pooled and evaporated. Before analyzing, the samples were re-suspended in 100 μl Diluent 1 and incubated over night at 4°C. The day of analysis the samples were vortexed and incubated for 10 min at 45°C, before the hormones were measured by use of the Delfia kit as mentioned above.
Ex vivo testosterone and progesterone production at GD 21 was determined by decapsulating and incubating the testis in a shaking water bath at 34°C for 3 h in DMEM/F12 medium containing 0.1 % BSA. Vials were centrifuged at 4000 x g for 10 min and the supernatants were stored at -80°C until hormone levels were analyzed as mentioned above. Testosterone levels were measured in undiluted supernatant, but for the measurement of the progesterone levels the supernatants were concentrated 5 times by the use of IST Isolute C18 SPE columns and resuspended in 100 μl Diluent 1.
2.2.2.7 Gene expression levels GD 21 and PND 16
The following genes were studied in testes at GD 21: scavenger receptor class B, member 1 (ScarB1), StAR, P450scc and Cyp17a1 (P450c17). In the testis ScarB1 transports serum lipoproteins (HDL) into the testis Leydig cell, where they are used in the synthesis of cholesterol. StAR transports the cholesterol from the outer to the inner mitochondrial membrane where the first enzyme in steroid synthesis, P450scc, is located, which converts cholesterol to pregnenolone. P450c17 is involved in several of the steps that convert pregnenolone to testosterone, and functions both as a 17α–hydroxylase and a 17,20-lyase (Felig et al., 1995) (Figure 3).
At PND 16 the following genes were studied in ventral prostate: AR, ODC, Compl. C3, IGF-1, PBP C3, and TRPM-2. In the epididymides the expression level of the placenta and embryos oncofetal gene (PEM) was quantified. PEM is a home-domain-containing protein, proposed to play a role in the regulation of androgen-dependent events in the epididymis.
Each sample was processed as previously described in section 2.2.1.2 and all genes were quantified from standard curves, and expression levels of each target gene were normalized to the expression level of the housekeeping gene 18S rRNA. See Table 2 for primer sequences used for the quantitative real-time PCR in testis and epididymis.
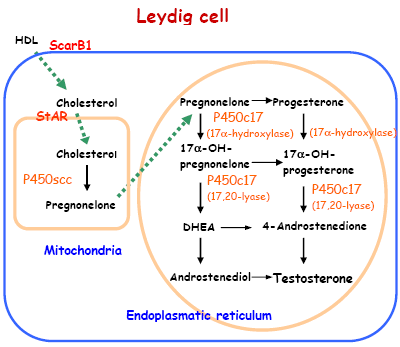
Figure 3 - Illustration of the pathway of testicular testosterone synthesis. The uptake of cholesterol and its conversion to testosterone involves numerous receptors and enzymes, including ScarB1, which is responsible for HDL uptake into Leydig cells, StAR that transport the cholesterol from the outer to the inner mitochondrial membrane and the steroid-converting enzymes P450scc and P450c17, which are involved in the conversion of cholesterol to testosterone.
2.2.2.8 Anogenital distance and nipple retention
AGD was measured in the offspring at birth (PND 1) using a stereomicroscope. On PND 13, the male pups were examined for the presence of areolas/nipples, described as a dark focal area (with or without a nipple bud) located where nipples are normally present in female offspring. To correlate for the size of the pups their body weights were measured at both time points (PND 1 and PND 13). AGD is expressed per cube root of body weight since this ratio has been demonstrated to be the most appropriate metric for normalizing of the data (Gallavan, Jr. et al., 1999).
2.2.2.9 Autopsy of offspring on PND 16
The external genitalia were inspected blinded to the observer at PND 16 in all males from all litters. The changes were scored on a scale from 1 to 4 in order to investigate whether male external genitals were feminized. The following criteria were used: Score 1: No effect: normal genital tubercle, the urethral opening is found at the tip of the genital tubercle and the preputial skin is intact. In the perineal area, thick fur extends caudally from the base of the genital tubercle half the distance to the anus. A furless area circumscribes the anus. Score 2: Mild dysgenesis of the external genitalia: A small cavity on the caudal surface of genital tubercle and a minor cleft in the preputial opening is observed, estimated 0.5-1.4 on an arbitrary scale. The furless area around anus expands towards the base of the genital tubercle, but thick fur is still present at the base of the genital tubercle. Score 3: Moderate dysgenesis of the external genitalia: the preputial cleft is larger, estimated 1.5-2.4 on an arbitrary scale. The urethral opening is situated half way down the inferior side of the genital tubercle (hypospadia). Lack of fur or thin fur in the perineal area ranging from the base of the genital tubercle and caudally to the furless area circumscribing the anus. Score 4: Severe dysgenesis of the external genitalia: The preputial cleft is large, estimated 2.5-3.5 on an arbitrary scale. The urethral opening is situated further than half way down the inferior side of the genital tubercle to the base of the genital tubercle. At the base of the genital tubercle a groove extending laterally is observed, and they are totally furless in the whole perineal area.
Table 2 - Sequences of the primer used for quantitative real-time PCR in testis and epididymis.
ScarB1
5’-FAM- AAA GCA TTT CTC CTG GCT GCG CAG-TAMRA-3’
Forward, 5’-TCT GGT GCC CAT CAT TTA CCA-3’
Reverse, 5’- AGC CCT TTT TAC TAC CAC TCC AAA-3’
StAR
5’-FAM- CTG ACT CCT CTA ACT CCT GTC TGC CTA CAT GGT-TAMRA-3’
Forward, 5’- CCC TTG TTT GAA AAG GTC AAG TG-3’
Reverse, 5’- TGA AAC GGG AAT GCT GTA GCT-3’
P450scc
5’-FAM- CCT TTA TGA AAT GGC ACA CAA CTT GAA GGT ACA-TAMRA-3’
Forward, 5’- ACG ACC TCC ATG ACT CTG CAA T-3’
Reverse, 5’- CTT CAG CCC GCA GCA TCT-3’
P450c17
5’-FAM- CGT CAA CCA TGG GAA TAT GTC CAC CAG A-TAMRA-3’
Forward, 5’-GCC ACG GGC GAC AGA A-3’
Reverse, 5’- CCA AGC CTT TGT TGG GAA-3’
PEM
5’-FAM- CCATCTATCAAGCTCCTCCCGCCACT-TAMRA-3’
Forward, 5’- CAT TTT GCT AAG CAG TGG TTC CT-3’
Reverse, 5’- CCT GCA CTC TGG ACA CAC TGA-3’
For the primer sequences used in the quantification of: 18S rRNA, PBP C3, ODC, TRPM-2, Compl.C3, IGF-1 and AR see Table 1.
2.2.2.10 Organ weight and histopathology PND 16
Body weights of all male pups were recorded. In one to two male per litter the following organs were excised and weighed: liver, kidneys, adrenals, testes, epididymides, seminal vesicles, ventral prostate, bulbourethal glands, and LABC. In the analysis of body and testis weight, generally one to four males per litter were used.
From one or two males per litter, the right or left testes were alternately fixed in Bouin’s fixative, paraffin embedded, and stained with hematoxylin and eosin. In one male per litter, the following organs were fixed in formalin: ventral prostate, seminal vesicles, and epididymides. All fixed organs were embedded in paraffin and examined by light microscopy after staining with hematoxylin and eosin and used for the histopathological evaluation.
In one to two females per litter, body weights were recorded. The thyroid, ovaries and uterus were excised and weighed from one female per litter.
2.2.2.11 Semen quality analysis PND 224
Due to toxicity in the highest dose groups, none of the high dose animals were available for the semen quality analysis. Semen quality was therefore only analyzed in the controls and the low dose groups of tebuconazole and epoxiconazole, using 11-12 male offspring per group at the age of approximately 7 months. The animals were anaesthetised by CO2/O2 and decapitated. The epididymis were removed and the cauda of the right epididymis was used for sperm motility analysis.
2.2.2.12 Sperm motility
The epididymis was trimmed of fat and cut at the corpus-cauda region. The cauda was placed in a petri dish containing 3 mL warm (37ºC) Medium 199, supplemented with 0.5% bovine serum albumin (crystallized and lyophilized; Sigma Chemicals Company, USA). Spermatozoa were obtained from the distal cauda where the tubular diameter was widest. Under the dissecting microscope, the cauda was held by forceps and several stabs were made into the tubules. The petri dish was placed in an incubator for 5 min. The cauda was removed and the sperm sample was diluted 10 times (in medium 199, supplemented with 0.5% bovine serum) and replaced in the incubator for 10 min, to allow dispersion of the spermatozoa. The sperm sample was loaded into a 100-µm flat cannula (HTR 1099, DIPL.ING. HOUM, Norway) and analyzed by computer assisted sperm analysis (CASA; HTM-IVOS version 10.6, Hamilton Thorne Research, Beverly, MA, USA). Several fields (minimum 20) were recorded at 60 Hz under 4´ dark field illumination, and the images were video recorded for later analysis.
The standard set-up was used during analysis and tracking errors were deleted through the edit and playback features. Twelve fields (minimum 200 sperm cells) were analyzed for each sperm sample. The parameters evaluated in this study were percent motile and percent progressive spermatozoa, curvilinear velocity (VCL), and amplitude of lateral head displacement (ALH), which describes the vigour of the spermatozoa, and progression parameters such as average path velocity (VAP), straight-line velocity (VSL), and straightness (STR).
2.3 Statistical analyses
2.3.1 In vitro and in vivo Hershberger test
Data did not deviate from normality or homogeneity of variance. Data were analyzed by one-way ANOVA followed by pair-wise comparisons between test and control groups using Dunnetts test. Significance was judged at p<0.05.
2.3.2 Developmental Study
The litter was generally considered the statistical unit and the alpha level was 0.05. The results were analyzed by analyses of variance (ANOVA), and in order to adjust for litter effects, litter was included in the analysis of variance as a nested factor.
For statistical evaluation of testosterone and progesterone levels in testes (2 - 4 males per litter) and ex vivo testosterone and progesterone production GD 21 (3 -11 males per litter), all males were included in the analysis. Data from one male and one female per litter at PND 16 (12-16 litters per group) and one or two males per litter at PND 224 (11-16 litters per group leading to a total of 16 animals per group) were used to analyze terminal body weight and organ weights. In order to adjust for litter effects, litter was included in the analysis of variance as an independent, random, and nested factor (proc mixed, SAS version 8, SAS Institute Inc, Cary, NC, USA). Organ weights were analyzed using treatment as one main factor and age as another main factor and body weight was used as a covariate. Non-processed and ln-transformed data were examined for normal distribution and homogeneity of variance. If an interaction between age groups and dose group was observed, the age groups were analyzed separately. When an overall significant treatment effect was observed, two-tailed comparison was performed using least square means. In cases where normal distribution and homogeneity of variance was not obtained, data were additionally tested with the non-parametric Wilcoxon Scores followed by a Kruskall-Wallis test.
Plasma hormone data were anayzed by a one-way ANOVA and, if significant, followed by Dunnett’s test. Significance was judged at p < 0.05. Sperm data were examined for normal distribution and homogeneity of variance. Single animal data were analyzed in a one-way analysis of variance (proc glm) followed by Dunnett`s t-test (version 8, SAS Institute Inc, Cary, NC, USA).
Version 1.0 October 2007, © Danish Environmental Protection Agency