Entomophthorales on cereal aphids
1. Introduction
| 1.1 | Background |
| 1.2 | Life cycle of Entomophthorales |
| 1.3 | Project objectives |
| 1.3.1 | Specific project objectives |
1.1 Background
Cereal aphids
Aphids (Homoptera: Aphididae) are among the most important pest insects in agriculture in the temperate climatic zones (Minks & Harewijn, 1988). Although more than 40 species of aphids are associated with cereal
(Vickerman & Wratten, 1979), only three species are of economic importance in Denmark: the English grain aphid Sitobion avenae (F.), the bird cherry-oat aphid Rhopalosiphum padi (L.) and the rose-grass aphid Metopolophium dirhodum (Walk.). In this report the three common species are designated cereal aphids.
Life cycle
Cereal aphids are holocyclic, which means that during a year both agame and gamogane generations occur. S. avenae are monoecious on cereal and grasses (Gramineae), and R. padi and M. dirhodum are heteroecious between bird cherry (Prunus padus) and Gramineae in the first case and roses and Gramineae in the second case (Vickerman & Wratten, 1979). All species overwinter in the egg stage and in spring fundatrices hatch from eggs and later they start to produce parthenogenetic offspring. For the two heteroecious species R. padi and M. dirhodum, an increasing number of individuals will develop wings and usually after two to three generations they will migrate from their primary (winter) to their secondary (summer) host (Dixon, 1973; Vickerman & Wratten, 1979; Hansen, 1995).
The emigrants colonise Gramineae on which several generations are produced (Dixon, 1973, Hansen 1995). On wheat, S. avenae prefers the upper leaves and then ears once these have emerged, M. dirhodum feeds on the leaf, and R. padi feeds primarily near the leaf bases and behind the leaf sheets (Dean, 1974). The relative abundance of the three species varies in both space and time (Hansen, 1995). When aphids are overcrowded, alate individuals develop and fly off to colonise other grass plants (Dixon, 1973; Vickerman & Wratten, 1979).
In autumn, alate gynoparae and alate males are produced in response to short day length and low temperature, and the gynoparae are always produced before the males (Dixon & Glen, 1971). R. padi and M. dirhodum fly to their primary host, while S. avenae remains on grasses where the gynoparae give birth to oviparae. After mating, the oviparous females lay the overwintering eggs (Dixon, 1973; Vickerman & Wratten, 1979; Hansen, 1995).
Natural enemies
A range of natural enemies such as predators, parasitoids and pathogens regulates aphid populations in cereal fields. Among the pathogens, all of the most prevalent and widely encountered species belong to the order Entomophthorales (Dean & Wilding, 1973; Dedryver, 1983; Feng et al., 1991). The species of entomophthoralean fungi identified from aphids belong to five genera: Conidiobolus, Entomophthora, Pandora, Neozygites and Zoophthora (Latgé & Papierok, 1988).
Epizootics caused by these fungi are often observed in cereals (Dean & Wilding, 1973; Dedryver, 1983; Feng et al., 1991; Steenberg & Eilenberg, 1995). Prevalence of infection may in some periods exceed 80%, indicating the possibility of utilising entomophthoralean fungi in microbial control of aphids either by developing a strategy for augmentation based on one of the fungi or by manipuling the environment so that the natural occurrence of fungi is favoured. Before augmentation or manipulation can be realized however, a better understanding of the epizootiology is necessary. However, most attention so far has been given to the effects of entomophthoralean fungi in cereal crops during the summer months. Thus knowledge concerning their effects during winter, spring and autumn and the possible interactions with other ecosystems is very limited.
1.2 Life cycle of Entomophthorales
Entomophthorales
The order Entomophthorales belongs to the subdivision Zygomycotina in the class Zygomycetes. Most Entomophthorales are pathogens to insects, however a few species are also saprophytes in soil.
Life cycle
In the aphid system all Entomophthorales have the same overall pattern of life cycle (figure 1.1). From the infected aphids, primary conidia are forcibly discharged. Primary conidia produce secondary conidia, which like the primary conidia are either forcibly discharged or are produced on long, slender conidiophores. Once the conidia land on a susceptible host under favourable conditions they will produce a germ tube that directly penetrates the insect cuticule (or first produce an appressorium and then penetrate the cuticle). Both enzymatic and physical processes are involved in the penetration. Once the fungus has penetrated the cuticle it will start to multiply and, after a period, the fungus will have invaded all the host tissues and the insect dies. The life cycle of the fungus can then follow one of two paths (1): An asexual path where conidiophores emerge through the insect integument and conidia are formed, or (2): A path where zygo- or azygospores (resting spores) are formed (Tanada & Kaya, 1993).
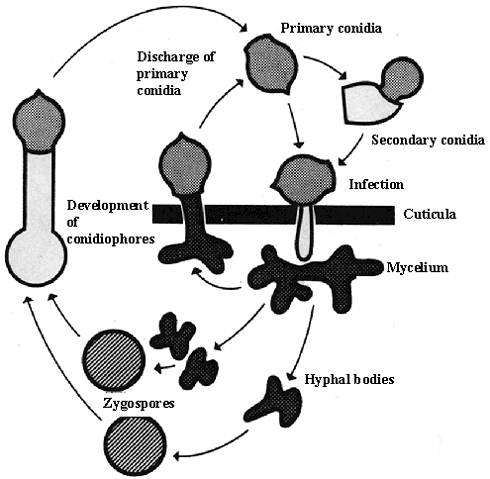
Figure 1.1
Generalised life cycle of Entomophthorales (modified after Eilenberg, 1983).
Epizootiology
Before infection of an aphid can occur, it is necessary with contact between the infective unit and the aphid. Tanada & Kaya (1993) reported that the spread of disease depends on both densities of the host and the infective unit. An increase in one or both pools enhances the probability for contact between conidia and aphids. In addition to the contact between conidia and aphids, suitable temperatures and relative humidities are necessary for sporulation, germination of conidia and for penetration of the aphid integument (Benz, 1987). Humidity and precipitation are often mentioned particularly as key factors (Missonnier et al., 1970; Dean & Wilding, 1971, 1973; Wilding 1975; Dedryver, 1983; Benz, 1987).
1.3 Project objectives
Project aim
The aim of the project was to clarify the potential of insect pathogenic fungi for better microbial control of cereal aphids.
Emphasis was on fungi belonging to the order Entomophthorales, particularly Pandora neoaphidis (Remaudière & Hennebert) Humber and S. avenae and
R. padi. A number of biological parameters have been investigated to assess which control strategy has the greatest potential. This report contains both the results of our experimental work and extracts from the literature.
1.3.1 Specific project objectives
Specific objectives
The specific objectives of this project were as follows:
| - | To implement and develop morphological, pathobiological and molecular characterisation methodologies for P. neoaphidis (chapter 2) |
| - | To describe the natural occurrence of Entomophthorales in different ecosystems relevant to cereal aphids (chapter 3) |
| - | To investigate the winter survival of entomophthoralean fungi infecting cereal aphids (chapter 4) |
| - | To implement and develop methods for in vivo and in vitro isolation and growth of Entomophthorales with emphasis on P. neoaphidis (chapter 5) |
| - | To investigate the virulence of P. neoaphidis against R. padi and S. avenae (chapter 6) |
| - | To describe the interactions between P. neoaphidis and cereal aphids, exemplified by a biological conceptual model of a system consisting of P. neoaphidis and S. avenae (chapter 7) |
| - | To evaluate the potential of Entomophthorales for controling aphids in cereals (chapter 8) |