Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products
3. Anionic surfactants
Anionic surfactants are surface-active compounds consisting of a hydrophobic alkyl chain
and a hydrophilic group. Anionic surfactants are negatively charged in aqueous solutions
due to the presence of a sulfonate, sulfate, carboxylate or phosphate group. Commercial
anionic surfactants contain mixtures of homologues with different alkyl chain lengths. For
some surfactant groups, the existence of different isomers also adds to the complex nature
and versatile application of these substances. The largest volume of anionic surfactants
is used in consumer products like, e.g., laundry detergents, cleaning and dishwashing
agents as well as personal care products. Another important application of anionic
surfactants includes cleaning agents designed for the industrial and institutional market.
By volume, the most important groups of anionic surfactants are fatty acid soaps, linear
alkylbenzene sulfonates, alkyl ether sulfates, and alkyl sulfates.
3.1 Alkyl sulfates
Alkyl sulfates (AS) are used in laundry detergents, frequently in combination with other anionic surfactants. Besides, AS are used in speciality products, including wool-washing agents, soap bars and liquid bath soaps, hair shampoos, and tooth pastes. Most of the AS used in consumer products are linear primary AS but some linear and branched secondary AS are also used (Painter 1992).
Primary AS have the structure:
![]()
Secondary AS have the structure:
![]()
The hydrophobic alkyl chain (R or R1 + R2) usually contains 12-18 carbon atoms. The sulfate group of secondary AS is found at all positions along the alkyl chain, except at the ends. The most widely used surfactant is the sodium salt, but raw materials with various other cations like, e.g., ammonium, magnesium, mono-, di-, tri-ethanolamine and cyclohexamine, are also produced.
3.1.1 Occurrence in the environment
Very few data on the concentration of AS in the environment could be found. The best basis for predicting the concentrations of AS in the aquatic environment is probably the data obtained in the monitoring program which was executed jointly by the Dutch Soap Association (NVZ) and the Dutch authorities. The monitoring showed that the concentrations of C12-15 AS in the effluent of seven representative municipal sewage treatment plants varied between 0.0012 and 0.012 mg/l with an average value of 0.0057 mg/l (Matthijs et al. 1999).
3.1.2 Environmental fateBiodgradation patjways
The biological degradation of AS is initiated by a hydrolytic cleavage of the sulfate ester bond catalysed by alkylsulfatases. The cleavage leaves inorganic sulfate and fatty alcohol which undergo oxidation by dehydrogenases to produce fatty acids via fatty aldehydes. The fatty acids are degraded by b -oxidation and finally totally mineralised or incorporated into biomass (Steber and Berger 1995). The biodegradation pathway for secondary AS differs from that of the primary AS by the formation of a ketone instead of an aldehyde. The ketone undergoes hydroxylation and forms an aldehyde and a carboxylic acid, which are further degraded by the b -oxidation. Biodegradation under anoxic conditions is anticipated to follow the same pathway as for the aerobic degradation (Steber and Berger 1995).
Effects of structure on biodegradability of A/S
Primary and secondary AS generally undergo complete primary biodegradation within a few days followed by a rapid ultimate biodegradation. Branched AS are also degraded quite rapidly, but multiple branchings of the alkyl chain considerably reduce the rate and extent of primary biodegradation (Swisher 1987; Painter 1992). The effect of branching was illustrated by a study in which the primary biodegradation was examined for a number of C12-15 AS with varying proportions of linear components. Primary biodegradation of anionic surfactants is usually quantified by measurements of methylene blue active substances (MBAS) which indicate a loss of surface-activity. The time required for the removal of 95% MBAS ranged from only 1 day for a coconut oil-AS containing 99% linear material, through 3 days for an oxo-AS containing 50% linear components, to as long as 12 days for an AS derived from tetra propylene containing less than 5% linear material (Painter 1992).
The ultimate aerobic biodegradability of AS was more or less unaffected of a 2-alkyl branching, and different structures with 2-alkyl branches of C1 (methyl-), C4 (butyl-) or C6 (hexyl-) were all readily degradable in the closed bottle test. Extensive branching of AS, as in a C13 propylene tetramer (4 internal CH3-groups; 10% quaternary carbons) and a C13 butylene trimer, however, may preclude compliance with the pass criteria for ready biodegradability (Battersby et al. 2000; Table 3.1).
Aerobic biodegradability
Rapid primary degradation of AS has frequently been reported for OECD tests, model sewage treatment systems, and seawater (Painter 1992; Steber and Berger 1995). There are numerous studies confirming the aerobic biodegradability of AS, and linear primary AS exceeds all other anionic surfactants in the rate of primary and ultimate biodegradation. Also secondary AS are normally readily biodegradable as, e.g., the oxygen uptake from biodegradation of a linear secondary C10-13 AS corresponded to 77% ThOD in 22 days. Some highly branched AS being poorly primary biodegradable may also resist ultimate biodegradation (Painter 1992). The fate of AS in wastewater treatment plants was illustrated in a model system using 14C-labelled C18 AS. At steady state, 60% of the added 14C was mineralized, 30% was associated with the sludge, and 10% was found in the effluent. About 90% of the 14C in the sludge was ascribed to bacterial biomass, and only 0.3% of the 14C found in the effluent was intact AS (Steber and Berger 1995). This indicates that AS are efficiently removed in wastewater treatment plants. Earlier studies have indicated that only 12-55% MBAS of a branched C13 AS was removed in activated sludge simulation tests (Painter 1992). Linear AS are readily biodegradable in the OECD 301 tests, whereas branching of the alkyl chain may lead to a less extensive ultimate biodegradability (Table 3.1).
Table 3.1
Ultimate aerobic biodegradability of AS.
AS |
Test |
Result |
Reference |
C12-18/C12-15-oxo |
Closed bottle test, 28 d Modified OECD screening test, 28 d Sturm test, 28 d |
63-95% ThOD
88-96% DOC
|
Schöberl et al. 1988 |
C12 branched (2-methyl, 2-butyl, 2-hexyl) |
Closed bottle test, 28 d |
85-100% ThOD |
Battersby et al. 2000 |
C12-14 |
Closed bottle test, 28 d Modified OECD screening test, 28 d |
90-94% ThOD
91% DOC |
Steber and Berger 1995 |
C13 branched (butylene trimer) |
Manometric respirometry test, 28 d |
50% ThOD |
Battersby et al. 2000 |
C13 branched (propylene tetramer, 4 internal CH3-groups, 10% quaternary carbons) |
Manometric respirometry test, 28 d |
37% ThOD |
Battersby et al. 2000 |
C14-15 branched |
BOD test, 30 d |
41% ThOD |
Kravetz et al. 1991 |
C14-15 |
BOD test, 30 d |
98% ThOD |
Kravetz et al. 1991 |
C15 branched (3 internal CH3-groups, quaternary carbon) |
Closed bottle test, 28 d |
0% ThOD |
Battersby et al. 2000 |
C16-18 |
Closed bottle test, 28 d Closed bottle test, 28 d Modified OECD screening |
77% ThOD
85-88% DOC |
Steber and Berger 1995
|
Anaerobic biodegradability
Anaerobic biodegradation of AS has been investigated in systems using digested sludge. A simple screening method, which was applied by Birch et al. (1989) and in the present study, determines the ultimate anaerobic biodegradability by measuring the gas production (i.e., CO2 and CH4) in sealed vessels containing diluted sludge (ECETOC 1988; ISO 1995). The test substance is added at a high concentration (e.g., 20-50 mg of carbon per litre) in order to measure the total net gas production from mineralization of the test substance. A drawback to the method is that the required concentration of test substance may inhibit the anaerobic bacteria and, hence, provide unfavourable conditions for biodegradation. On the other hand, the bacterial community in the digested sludge may be better adapted to biodegradation of man-made chemicals than the bacteria in natural habitats. The possibilities for predicting the fate in anoxic environments from results obtained in the screening tests have not yet been evaluated. Both linear and 2-alkyl-branched primary AS are degraded to a high extent under anaerobic conditions (Table 3.2).
Table 3.2
Ultimate anaerobic biodegradability of AS in digested sludge.
AS |
Type of test and duration |
Result |
Reference |
C12 |
Measurement of 14CH4 and 14CO2 evolution, 28 d |
> 90% ThCH4 + ThCO2 |
Steber and Berger 1995 |
C12-14 |
Measurement of gas production, 35° C, 40-50 d |
77-84% ThCH4 |
Salanitro and Diaz 1995 |
Measurement of gas production, 35° C, 56 d |
85% ThGP |
This study (Appendix; Table A7, Figure A7) |
|
C14 |
Measurement of 14CH4 and 14CO2 evolution, 15 d |
80% ThCH4 + ThCO2 |
Nuck and Federle 1996 |
C14-15 (20% branched) |
Measurement of gas production, 35° C, 40-50 d |
65-78% ThCH4 |
Salanitro and Diaz 1995 |
C18 |
Measurement of 14CH4 and 14CO2 evolution, 28 d |
> 90% ThCH4 + ThCO2 |
Steber and Berger 1995 |
Measurement of gas production, 35° C, 56 d |
88% ThGP |
Birch et al. 1989 |
The anaerobic gas production test of C12-14 AS in the present study was
conducted by using an inoculum concentration of 1.0 g digested sludge dry weight per litre
of test medium as described in the ISO 11734 method. The ultimate biodegradation of the AS
attained 20% of ThGP after 28 days, whereas 85% was reached after 56 days (Table 3.2;
Appendix). The study of Salanitro and Diaz (1995) was based on a linear C12-14
AS and a C14-15 AS composed of 80% linear alcohols and 20% 2-alkyl-branched
alcohols. The fact that biodegradation of both the linear and the branched part of the C14-15
AS occurred was confirmed by a recovery of methane which approached 100% in tests using a
relatively low concentration (10 mg/l) of the substrate. Wagener and Schink (1987) showed
that C12 AS was degraded to CO2 and methane during anaerobic
incubation with digested sludge as well as with creek sludge.
Bioaccumulation
Bioaccumulation of AS in aquatic organisms has been determined in tests with goldfish, rainbow trout, carp and guppy. The majority of these experiments has been performed with radiolabelled compounds, mainly 35S-labelled AS, which do not allow a distinction between parent AS and metabolites. As the AS is metabolised in the organism, the bioconcentration factor for the intact surfactant may be overestimated in experiments using radiolabelling techniques instead of chemical analyses. Whole body BCF values, as well as specific tissue BCF values, have been determined in fish for AS between C12 and C16 (Table 3.3).
Table 3.3
Whole body BCF values in fish.
AS |
Species |
Uptake/ |
BCF |
Reference |
C12 |
Goby |
240 h/- |
7.15 |
Topcuoglu and Birol 1982 |
C12 |
Carp |
72 h/120 h |
2.1 |
Wakabayashi et al. 1980 |
C14 |
Carp |
72 h/120 h |
11 |
Wakabayashi et al. 1980 |
C16 |
Carp |
72 h/120 h |
73 |
Wakabayashi et al. 1980 |
The BCF values obtained with C14 AS and C16 AS (Wakabayashi et
al. 1980; Table 3.3) are both considered invalid as steady state conditions were not
obtained during the experiment. BCF values up to 2,200 for C12 AS have been
determined in the gall bladder of goldfish, Carassius auratus (Tovell et al.
1975). The high concentrations of radiolabelled material that are frequently found in the
gall bladder are interpreted as the result of biotransformation in the liver and
subsequent excretion of metabolites in the gall bladder (Comotto et al. 1979;
Wakabayashi et al. 1987; Goodrich et al. 1991; Toshima et al. 1992).
The common experimental condition where the fish are not fed during the exposure to the
test substance may further increase the accumulation of radiolabelled substances in the
gall bladder. When fish do not eat, the content of the gall bladder is not emptied into
the gut, and high concentrations of metabolites may accumulate in the gall bladder.
Wakabayashi et al. (1980) found that the uptake and elimination of C12,
C14, and C16 AS were rapid and that these surfactants were
metabolised to more polar compounds in the fish. As the BCF values for AS (Table 3.3) are
possibly overestimated due to the use of radiolabelled compounds, AS are generally
considered to have a low potential for bioconcentration in aquatic organisms.
3.1.3 Effects on the aquatic environment
The aquatic toxicity of AS seems to increase with increasing alkyl chain length. This has been shown for daphnids and for some fish species. An overall comparison of the acute toxicity between the primary and secondary AS shows only minor differences in the toxicity, although only a few studies for comparison are available.
Algae
The available data describing the toxicity of AS towards algae indicate that the lowest EC50 values range between 1 and 10 mg/l for C12 AS (Table 3.4).
Table 3.4
Effects of AS to algae.
Species |
AS |
EC50 |
Test duration |
Reference |
Selenastrum capricornutum |
Na-C12 |
4 |
|
Painter 1992 |
Pseudoiosochrysis paradoxa |
Na-C12 |
1.31 |
|
Roberts et al. 1982 |
Skeletonema costatum |
Na-C12 |
2.31 |
|
Roberts et al. 1982 |
Prorocentrum minimum |
Na-C12 |
1.31 |
|
Roberts et al. 1982 |
Skeletonema costatum |
C12-14 |
27 |
72 h |
Verge et al. 1996 |
Microcosmos |
C12 |
NOEC: > 0.55* |
28 d |
Belanger and Rupe 1996 |
1
Test based on assimilation of 14C-NaHCO3.* Effect concentration based on measured concentrations.
Invertebrates
The toxicity of AS towards invertebrates has mainly been examined in tests with Daphnia magna. Lundahl and Cabridenc (1978) showed that the acute toxicity of AS to Daphnia magna increased with increasing alkyl chain length (Table 3.5). It has been shown that during degradation of C12 AS, the toxicity first increased to a maximum after 30 hours and then fell to almost a negligible value. The increase in toxicity was explained by the formation of the more toxic dodecanoic acid which is rapidly transformed to other and less toxic metabolites (Painter 1992).
Table 3.5
Effects of AS to invertebrates.
Species |
AS |
EC50/LC50 |
Test duration |
Reference |
Daphnia magna |
C4 |
8,200 |
24 h |
Lundahl and Cabridenc 1978 |
Daphnia magna |
C8 |
4,350 |
24 h |
Lundahl and Cabridenc 1978 |
Daphnia magna |
C9 |
2,300 |
24 h |
Lundahl and Cabridenc 1978 |
Daphnia magna |
C10 |
800 |
24 h |
Lundahl and Cabridenc 1978 |
Daphnia magna |
C12 |
80 |
24 h |
Lundahl and Cabridenc 1978 |
Daphnia magna |
C13 |
42 |
24 h |
Lundahl and Cabridenc 1978 |
Daphnia magna |
C12 |
1.8 |
48 h |
Bishop and Perry 1979 |
Daphnia magna |
C12 |
10.8-13.5 |
48 h |
Lewis and Horning 1991 |
Acartia tonsa |
Na-C12 |
0.6 |
96 h |
Roberts et al. 1982 |
Mesocosmos |
C12 |
LOEC: 0.58* |
56 d |
Belanger et al. 1995 |
* Effect concentration based on measured concentrations.
Fish
The toxicity of AS to fish has been demonstrated to increase with increasing alkyl chain length as also seen in studies with Daphnia magna. Studies performed by Kikuchi et al. (1976) showed that the 24 h-LC50 values for killifish in distilled water decreased by a factor of about 10 when the alkyl chain was increased by two carbon atoms. C16 was 10 times more toxic than C14, which was about 10 times more toxic than C12 (Table 3.6). Differences between the toxicity values for AS with similar chain lengths may be due to different species, but are probably also a result of different times of exposure and hardness of water (Painter 1992).
Table 3.6
Effects of AS to fish.
Species |
AS |
LC50 |
Test duration |
Reference |
Carp (Cyrinus carpio) (prelarvae) |
C10 |
13 |
48 h |
Kikuchi et al. 1976 |
Carp (prelarvae) |
C12 |
13 |
48 h |
Kikuchi et al. 1976 |
Carp (prelarvae) |
C14 |
5.0 |
48 h |
Kikuchi et al. 1976 |
Carp (prelarvae) |
C16 |
0.69 |
48 h |
Kikuchi et al. 1976 |
Bluegill sunfish (Lepomis macrochirus) |
C12 |
4.5 |
96 h |
Painter 1992 |
Rice fish (Oryzias latipes) |
C12 |
51 |
48 h |
Kikuchi et al. 1976 |
Rice fish |
C14 |
5.9 |
24 h |
Kikuchi et al. 1976 |
Rice fish |
C16 |
0.50 |
48 h |
Kikuchi et al. 1976 |
Minnow (Phoxinus phoxinus) |
C12 |
30.5 |
24 h |
Lundahl and Cabridenc 1978 |
Sheepshead minnow (Cyprinodon variegatus) |
C12 |
4.1 |
96 h |
Roberts et al. 1982 |
Atlantic silverside (Menida menida) |
C12 |
2.8 |
96 h |
Roberts et al. 1982 |
Carp |
C12 |
18 (Egg hatching) |
- |
Kikuchi et al. 1976 |
Carp |
C14 |
2.9 (Egg hatching) |
- |
Kikuchi et al. 1976 |
Carp |
C16 |
> 1.6 (Egg hatching) |
- |
Kikuchi et al. 1976 |
Sediment organisms
Whereas most correlations between AS structure and toxicity show an increasing toxicity with increasing alkyl chain length, the budding in Hydra attenuata was apparently more affected by C10 AS than by C12, C14, and C16 AS (Bode et al. 1978) (Table 3.7). The authors suggested that the decrease in toxicity with increasing alkyl chain length was attributable to reduced solubility in water.
Table 3.7
Effects of AS to sediment-living organisms.
Species |
AS |
EC50/LC50 |
Test duration |
Reference |
Hydra attenuata |
C10 |
55 |
24 h |
Bode et al. 1978 |
Hydra attenuata |
C12 |
58 |
10 d |
Bode et al. 1978 |
Hydra attenuata |
C14 |
NOEC:63 |
10 d |
Bode et al. 1978 |
Hydra attenuata |
C16 |
LOEC: 688 |
10 d |
Bode et al. 1978 |
Arenicola marina |
C12 |
15.2 |
48 h |
Painter 1992 |
Tresus carpax (larvae) |
C12 |
0.35 |
48 h |
Painter 1992 |
Crassostrea gigas (larvae) |
C12 |
0.70-1.16 |
48 h |
Cardwell et al. 1977 |
Crassostrea gigas (larvae) |
C12 |
1.0 |
48 h |
Cardwell et al. 1978 |
3.1.4 Effects on human health
Toxicokinetics and acute toxicity
AS are readily absorbed from the gastrointestinal tract after oral administration. Penetration of AS through intact skin appears to be minimal (IPCS 1996). AS are extensively metabolized in various species resulting in the formation of several metabolites. The primary metabolite is butyric acid–4–sulfate. The major site of metabolism is the liver (Gloxhuber and Künstler 1992; IPCS 1996). AS and their metabolites are primarily eliminated via the urine and only minor amounts are eliminated via the faeces. In rats about 70–90% of the dose was eliminated via the urine within 48 hours after oral, intravenous or intraperitoneal administration of 1 mg of AS per rat (Burke et al. 1975). The acute toxicity of AS in animals is considered to be low after skin contact or oral intake (Table 3.8).
Table 3.8
Acute toxicity (LD50) of AS.
AS |
Species |
Route |
LD50 (mg/kg body weight) |
Reference |
Various |
Rat |
Oral |
5,000-15,000 |
Kirk-Otmer 1994 |
Various |
Rat |
Oral |
1,000-11,000 |
Falbe 1986; Gloxhuber and Künstler 1992; |
C6-18 |
Mouse |
Oral |
2,200 - < 8,000 |
Gloxhuber and Künstler 1992 |
C12 |
Rat |
Oral |
1,200 |
Gloxhuber and Künstler 1992 |
C12 |
Rat |
Oral |
1,000 – 2,700 |
Singer and Tjeerdema 1993 |
Skin and eye irritation
For a homologous series of AS (C8 to C16), maximum swelling of stratum corneum (the outermost layer of epidermis) of the skin was produced by the C12 homologue. This is in accordance with the fact that the length of the hydrophobic alkyl chain influences the skin irritation potential. Other studies have shown that especially AS of chain lengths C11, C12 and C13 remove most amino acids and soluble proteins from the skin during washing (Prottey and Ferguson 1978; Rhein et al. 1986). Concentrated samples of AS are skin irritants in rabbits and guinea pigs. AS are non-irritant to laboratory animals at a 0.1% concentration (Gloxhuber and Künstler 1992). C12 AS is used in research laboratories as a standard substance to irritate skin and has been shown to induce an irritant eczema (Frankild 1992). AS were found, by many authors, to be the most irritating of the anionic surfactants, although others have judged the alkyl sulfates only as irritant as laurate (fatty acid soap) (Tupker 1990).
A structure/effect relationship with regard to the length of the alkyl chain can also be observed on mucous membranes. The maximum eye irritation occurs at chain lengths of C10 to C14 (Falbe 1986). In acute ocular tests, 10% C12 AS caused corneal damage to the rabbit eyes if not irrigated (Davies et al. 1976). Another study showed that a 1.0% aqueous C12 AS solution only had a slight effect on rabbit eyes, whereas 5% C12 AS caused temporary conjunctivitis, and 25% C12 AS resulted in corneal damage (Singer and Tjeerdema 1993).
Subchronic toxicity
In a 13-week feeding study, rats were fed dietary levels of 0, 40, 200, 1,000 or 5,000 ppm of C12 AS. The only test material related effect observed was an increase in absolute organ weights in the rats fed with the highest concentration which was 5,000 ppm. The organ weights were not further specified and no other abnormalities were found (Walker et al. 1967).
Mutagenicity and carcinogenicity
In a mutagenicity study, rats were fed 1.13 and 0.56% C12 AS in the diet for 90 days. This treatment did not cause chromosomal abberations in the bone marrow cells (Hope 1977). Mutagenicity studies with Salmonella typhimurium strains (Ames test) indicate no mutagenic effects of C12 AS (Mortelmans et al. 1986). The available long-term studies in experimental animals (rats and mice) are inadequate to evaluate the carcinogenic potential of AS. However, in studies in which animals were administered AS in the diet at levels of up to 4% AS, there was no indication of increased risk of cancer after oral ingestion (Falbe 1986; IPCS 1996).
Reproductive toxicity
No specific teratogenic effects were observed in rabbits, rats or mice when pregnant animals were dosed with 0.2, 2.0, 300 and 600 mg C12 AS/kg body weight/day by gavage during the most important period of organogenesis (day 6 to 15 of pregnancy for mice and rats and day 6 to 18 of pregnancy for rabbits). Reduced litter size, high incidence of skeletal abnormalities and foetal loss were observed in mice at 600 mg C12 AS/kg/day, a dose level which also caused severe toxic effects in the parent animals in all three species (Palmer et al. 1975a; Singer and Tjeerdema 1993). An aqueous solution of 2% AS was applied (0.1 ml) once daily to the dorsal skin (2 x 3 cm) of pregnant mice from day 1 to day 17 of gestation. A solution of 20% AS was tested likewise from day 1 to day 10 of gestation. The mice were killed on days 11 and 18, respectively. A significant decrease in the number of implantations was observed when mice were treated with 20% AS compared to a control group which was dosed with water. No evidence of teratogenic effects was noted (Nomura et al. 1980).
When aqueous solutions of 2% and 20% AS (0.1 ml) were applied once per day to the dorsal skin (2 x 3 cm) of pregnant ICR/Jc1 mice from day 12 to day 17 of gestation no effects on pregnancy outcome were detected. Treatment with 20% AS resulted in growth retardation of suckling mice, but this effect disappeared after weaning (Nomura et al. 1980). A 10% AS solution (0.1 ml) was applied twice daily to the dorsal skin (2 x 3 cm) of pregnant lCR/Jc1 mice during the preimplantation period (days 0-3 of gestation). A significant number of embryos collected on day 3 as severely deformed or remained at the morula stage. Nomura et al. (1980) reported that the number of embryos in the oviducts was significantly greater for the mice dosed with AS as compared to the control mice. No pathological changes were detected in the major organs of the dams.
Classification
AS are generally classified according to Comité Européen des Agents de Surface et leurs Intermédiaires Organiques (CESIO) as Irritant (Xi) with the risk phrases R38 (Irritating to skin) and R41 (Risk of serious damage to eyes). An exception has been made for C12 AS which is classified as Harmful (Xn) with the risk phrases R22 (Harmful if swallowed) and R38 and R41 (CESIO 2000).
AS are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
3.2 Alkyl ether sulfates
Alkyl ether sulfates (AES), or alkyl ethoxy sulfates, are being used increasingly, frequently in combination with other anionic and nonionic surfactants, in liquid bath soaps, hair shampoos, and mechanical dishwashing agents. Besides, AES are important as ingredients in industrial cleaning agents and as auxiliaries in some industrial process steps (Steber and Berger 1995). AES are primary sulfate esters manufactured from the corresponding alcohol ethoxylates.
AES have the following structure(s):
![]()
Alkyl ether sulfates: R2 = H; R1 = C10-14; n = 1-4
Oxoalkyl ether sulfates: R2 = H, C1, C2; R1 +
R2 = C11-15; n = 1-4
The structures above describe the normal alkyl chain length for AES, but sometimes longer alkyl or ethoxylate chains are seen.
3.2.1 Occurrence in the environment
Very few data on the concentration of AES in the environment have been found. The monitoring conducted in the Netherlands showed that the concentrations of C12-15 AES in the effluent of seven representative municipal sewage treatment plants varied between 0.003 and 0.012 mg/l with an average value of 0.0065 mg/l (Matthijs et al. 1999).
3.2.2 Environmental fateBiodegradation pathways
The most frequent initial step in the biodegradation of AES is the cleavage of an ether bond (Steber and Berger 1995). The cleavage may take place at any ether bond producing a fatty alcohol or an alcohol ethoxylate and ethylene glycol sulfates of various lengths. The alcohol is degraded by w /b -oxidation, whereas the ethylene glycol sulfate is eliminated stepwise by oxidation and cleavage of C2-units along with a desulfation (Steber and Berger 1995). The ether cleavage and the desulfation may also take place in the absence of molecular oxygen, but the anaerobic biodegradation pathway has not yet been verified (Steber and Berger 1995).
Effects of structure on biodegradability of AES
The length of the alkyl chain and the number of EO units apparently do not affect the degree of aerobic biodegradation, but branching of the alkyl chain may hinder the primary biodegradation of AES. E.g., according to studies reported by Painter (1992), the removal of MBAS was 97% for a linear primary AES, 90% for a linear primary oxo-AES, and 50% for a branched tetra-propylene based primary AES during 3 days.
Aerobic biodegradability
AES are degraded readily and completely under aerobic conditions. E.g., for C12-14 AE3S, a rapid primary degradation of 90-100% is reported to take place within a period of 1 to 5 days (Painter 1992). In activated sludge simulation tests 67-99% DOC was removed by degradation of C12-14 AE2S and C12-15 AE3S (Schöberl et al. 1988). The ultimate biodegradation of AES has been confirmed in OECD 301 tests for ready biodegradability (Table 3.9).
Table 3.9
Ultimate aerobic biodegradability of AES.
AES |
Test |
Result |
Reference |
C12-14 AE2S |
Closed bottle test, 28 d |
58-100% ThOD |
Schöberl et al. 1988 |
C12-15 oxo-AE3S |
Modified OECD screening test, 28 d |
96-100% DOC |
Schöberl et al. 1988 |
CO2 evolution test, 28 d |
65-83% ThCO2 |
Schöberl et al. 1988 |
|
C12-18 AE8.5S |
Closed bottle test, 28 d |
100% ThOD |
Steber and Berger 1995 |
Anaerobic biodegradability
The primary of AES has been confirmed in early studies in which a removal of 64% MBAS for C12-14 AE3S (in 28 days) and 70% MBAS for C16 AE1S (in 17 days) were observed (Painter 1992). The ultimate anaerobic biodegradability of C12 AE3S was examined in gas production screening tests using either digested sludge, a marine sediment or material from a freshwater swamp as inoculum. The 20 mg of AES carbon per litre which was applied in these tests proved to be inhibitory to the anaerobic bacteria, and only in the digested sludge a net gas production corresponding to 23% ThGP was observed during 56 days (Madsen et al. 1996a). Experiments using a higher inoculum to test substrate ratio have shown that extensive biodegradation of AES may occur under anoxic conditions. Nuck and Federle (1996) examined the anaerobic degradation of a C14 AE3S which was 14C-labelled in the ethoxylate moiety. By using an inoculum of 24-29 g of digester sludge per litre of medium, the recovery of 14CO2 and 14CH4 equalled 88.4% (1 mg AES/l) and 87.6% (10 mg AES/l) after 17 days of incubation at 35oC.
Bioccumulation
The uptake, distribution and elimination of 35S labelled C12 AE3S and C12 AE5S have been investigated in carp (Cyprinus carpio) without distinction between parent AES and metabolites (Kikuchi et al. 1980). The following BCF values for the two substances, respectively, were determined: Whole body, 18 and 4.7; gall bladder, 3,400 and 940; and hepatopancreas, 46 and 18. Both the uptake and the elimination were reported to be rapid. Due to metabolisation of AES in the organism, the BCF for the intact surfactant may be overestimated in experiments using radiolabelled compounds. For the whole body, as well as for the gall bladder, the steady state was not reached within 72 hours and, hence, the reported BCF values are considered to be invalid. Furthermore, the fish were not fed during the study. The high concentrations found in the gall bladder are thus most probably due to biotransformation of AES in the liver and subsequent excretion of radiolabelled metabolites in the gall bladder (Comotto et al. 1979; Wakabayashi et al. 1987; Goodrich et al. 1991; Toshima et al. 1992). Based on the studies above, AES are not considered to bioconcentrate in aquatic organisms.
3.2.3 Effects on the aquatic environment
The chemical structure of AES highly influences the effect on aquatic organisms. The relations between alkyl chain length, number of EO groups and toxicity are complex and not yet resolved, but in general, changes in EO numbers affects toxicity more than changes in the alkyl chain length. In AES with alkyl chains of less than C16, the toxicity tended to decrease with increasing numbers of EO, but this was reversed for alkyl chain lengths above C16. The toxicity of AES thus seems to peak at alkyl chain lengths of C16. In a study of the acute toxicity of various AES (C8 to C19.6 and 1-3 EO) to bluegill sunfish (Lepomis macrochirus), the LC50 fell from > 250 mg/l for C8 and 375 mg/l for C10 to 24 mg/l for C13, 4-7 mg/l for C14, 2 mg/l for C15 and 0.3 mg/l for C16, and then increased to 10.8 mg/l for C17.9 and 17 mg/l for C19.6 (Little 1981).
Algae
Not very many and mainly quite old data describing the effects of AES towards algae were found in the literature. Besides the effect concentrations presented in Table 3.10, Kutt and Martin (1974) reported very low toxicity values for the marine red tide dinoflagellate, Gymnodium breve, when this species was exposed to coconut ethoxylate sulfate. The authors observed 87%, 63% and 44% inhibition at 0.0025; 0.0125 and 0.05 mg/l, respectively, after 48 hours of exposure. Experiments in which Gymnodium breve was exposed with LAS confirm that this species is highly sensitive to surfactants (Hitchcock and Martin 1977), and occasionally available data for Gymnodium breve should therefore not be used for comparison of the aquatic toxicity between various surfactants. Typical EC50 values describing the toxicity of AES towards algae vary between 4 and 65 mg/l (Table 3.10). In a microcosmos study performed by Belanger et al. (1996), the NOEC values appeared to be above the concentrations tested.
Table 3.10
Effects of AES to algae.
Species |
AES |
EC50 (mg/l) |
Test duration |
Reference |
Selenastrum capricornutum |
C10-15 AE3S |
65 |
48 h |
Yamane et al. 1984 |
Selenastrum capricornutum |
C12-14 AEnS |
20 (97% inhibition of growth) |
21 d |
Nyberg 1988 |
Selenastrum capricornutum |
C10-16 AE2S |
30 (91% inhibition of growth) |
21 d |
Nyberg 1988 |
Selenastrum capricornutum |
AES |
65 |
72 h |
Fendinger et al. 1994 |
Selenastrum capricornutum |
C12-14 AES |
32 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
Cx AE9S |
4-8 |
- |
Painter 1992 |
Nitzschia fonticula |
Cx AE9S |
5-10 |
- |
Painter 1992 |
Microcystis aeruginosa |
Cx AE9S |
10-50 |
- |
Painter 1992 |
MicrocosmosAlgae community |
C14.5 AES |
NOEC: 0.61* |
28 d |
Belanger et al. 1996 |
* Effect concentrations based on measured concentrations.
Invertebrates
Painter (1992) reported ranges for EC50 for the acute toxicity of AES to daphnids between 1 and 50 mg/l. However, an EC50 of 0.37 mg/l was observed in a 21-day reproduction test with Daphnia magna (Maki 1979). Also Belanger et al. (1995) observed very low effect concentrations of AES on invertebrates as both mayfly and bivalve populations were impaired at 0.77 mg/l during an 8-week mesocosmos study (Table 3.11).
Table 3.11
Effects of AES to invertebrates.
Species |
AES |
EC50/LC50 |
Test duration |
Reference |
Daphnia magna |
C13.67 AE2.25S |
1.17* (0.82-1.66)** |
96 h |
Maki 1979 |
Daphnia magna |
C13.67 AE2.25S |
0.74* |
21 d |
Maki 1979 |
Daphnia magna |
C13.67 AE2.25S |
0.37* |
21 d |
Maki 1979 |
Mesocosmos |
C14-15 AE2.17S |
LOEC: 0.77* |
56 d |
Belanger et al. 1995 |
*Effect concentrations based on measured concentrations.
** 95% confidence intervals.
Fish
The LC50 values for fish are in the range between 0.39 to 450 mg/l (Table 3.12). A LOEC value of 0.22 mg/l has been reported for a chronic life cycle test with a duration of 1 year (Maki 1979). The toxicity of AES towards fish seems to increase with increasing alkyl chain length for AES with up to 16 carbons.
Table 3.12
Effects of AES to fish.
Species |
AES |
LC50 (mg/l) |
Test duration |
Reference |
Fathead minnow (Pimephales promelas) |
C11 AE4S |
17.0 |
24 h |
Painter 1992 |
Fathead minnow |
C12 AE2S |
1.5 |
24 h |
Painter 1992 |
Fathead minnow |
C14 AE2S |
1.8 |
24 h |
Painter 1992 |
Fathead minnow |
C16 AE2S |
1.0 |
24 h |
Painter 1992 |
Fathead minnow |
C18 AE2S |
80 |
24 h |
Painter 1992 |
Fathead minnow |
C14 AE4S |
4.0 |
24 h |
Painter 1992 |
Fathead minnow |
C16 AE4S |
0.9 |
24 h |
Painter 1992 |
Fathead minnow |
C18 AE4S |
15 |
24 h |
Painter 1992 |
Fathead minnow |
C14 AE6S |
9.3 |
24 h |
Painter 1992 |
Fathead minnow |
C16 AE6S |
0.8 |
24 h |
Painter 1992 |
Fathead minnow |
C18 AE6S |
2.1 |
24 h |
Painter 1992 |
Rainbow trout |
C9-10 AE2.5S |
400-450 |
96 h |
Painter 1992 |
Rainbow trout |
C12-13 AE2S |
28 |
96 h |
Painter 1992 |
Rainbow trout |
C12-15 AE3S |
8.9 |
96 h |
Painter 1992 |
Brown trout |
C12-15 AE3S |
1.0-2.5 |
96 h |
Reiff et al. 1979 |
Harlequin Fish |
C12-15 AE3S |
3.9 |
48 h |
Reiff et al. 1979 |
Golden orfe |
C12-15 AE3S |
3.95 |
48 h |
Reiff et al. 1979 |
Fathead minnow, fry |
C14-16 AE2,25S |
0.63 |
45 d |
Little 1981 |
Fathead minnow, juvenile |
C14-16 AE2,25S |
0.94 |
45 d |
Little 1981 |
Fathead minnow |
C13.7 AE2.25S |
LOEC: 0.22* |
365 d |
Maki 1979 |
Sheepshead minnow (Cyprinodon variegatus) |
C14-16 AE2.25S |
0.39 |
45 d |
Little 1981 |
* Effect concentrations based on measured concentrations.
Toxicokinetics and acute toxicity
AES are easily absorbed in the intestine in rats and humans after oral administration. Radiolabelled C11 AE3S and C12 AE3S were extensively metabolized in rats and most of the 14C-activity was eliminated via the urine and expired air independently of the route of administration (oral, intraperitoneal or intravenous). The main urinary metabolite from C11 AE3S is propionic acid-3-(3EO)-sulfate. For C12 and C16 AE3S, the main metabolite is acetic acid-2-(3EO)-sulfate. The alkyl chain appears to be oxidized to CO2 which is expired. The EO-chain seems to be resistant to metabolism (McDermott et al. 1975; Taylor et al. 1978). Only small amounts of non-specified AES were absorbed through the skin (Painter 1992). The LD50 values after oral administration of AES range from 1,000 – 5,000 mg/kg body weight for rats (Falbe 1986; Gloxhuber and Künstler 1992; Painter 1992) indicating a low acute toxicity.
Skin and eye irritation
AES are better tolerated on the skin than, e.g., alkyl sulfates and it is generally agreed that the irritancy of AES is lower than that of other anionic surfactants. Alkyl chain lengths of 12 carbon atoms are considered to be more irritating to the skin compared to other chain lengths (Tupker 1990; Gloxhuber and Künstler 1992). The skin irritating properties of AES normally decrease with increasing level of ethoxylation (Falbe 1986; KEMI 1990). Undiluted AES should in general be considered strongly irritating. Even at concentrations of 10% moderate to strong effects can be expected. However, only mild to slight irritation was observed when a non-specified AES was applied at 1% to the skin (SFT 1991).
Subchronic and long term toxicity
A 90-day subchronic feeding study in rats with 1% of AE3S or AE6S with alkyl chain lengths of C12-14 showed only an increased liver/body weight ratio (Scailteur et al. 1986). In a chronic oral study with a duration of 2 years, doses of C12-AE3S of 0.005 – 0.05% in the diet or drinking water had no effects on rats. The concentration of 0.5% sometimes resulted in increased kidney or liver weight (Falbe 1986; Scailteur et al. 1986; Painter 1992).
Carcinogenicity
There is no indication of increased risk of cancer after oral ingestion of AES. Carcinogenic effects were not observed after skin application (Falbe 1986; SFT 1991).
Reproductive toxicity
No evidence of reproductive and teratogenic effects was seen in a two-generation study in rats fed with a mixture (55:45) of AES and linear alkylbenzene sulfonates. Dietary levels of 0.1, 0.5, and 1% were administered to the rats either continuously or during the period of major organogenesis during six pregnancies. No changes in reproductive or embryogenic parametres were observed (Nolen et al. 1975).
Classification
AES are generally classified according to Comité Européen des Agents de Surface et leurs Intermédiaires organiques (CESIO) as Irritant (Xi) with the risk phrases R38 (Irritating to skin) and R36 (Irritaing to eyes). An exception has been made for AES (2-3E0) in a concentration of 70-75% where R36 is substituted with R41 (Risk of serious damage to eyes).
AES are not included in Annex 1 of the list of dangerous substances of Council Directive 67/548/EEC.
3.3 Linear alkylbenzene sulfonates
Linear alkylbenzene sulfonates (LAS) are, by volume, the most important group of synthetic anionic surfactant today. LAS are mainly used in laundry detergents and cleaning agents. LAS are frequently used as the sodium salts as the sole surfactant in a formulation or in conjunction with other anionic, nonionic or cationic surfactants. LAS consist of an alkyl chain attached to a benzene ring in the para position to the sulfonate group. Sometimes toluene, xylene and naphthalene are used in place of benzene. The homologue distribution in commercial products covers alkyl chain lengths from C10 to C13 with an average chain length of C11.6. LAS raw materials are derived from linear alkyl benzenes in which the ring is attached to a C-atom which is itself attached to two other C-atoms. The benzene ring may be attached to any of the C atoms from C2 to C6 but not to C1. Structures in which the benzene ring may be attached to different C atoms are described as isomers. E.g., the structure with a C12 alkyl chain and the benzene ring attached at the second alkyl carbon is designated as the C12-2-isomer and abbreviated C12-2.
LAS have the following structure:
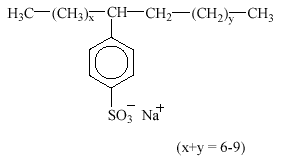
3.3.1 Occurrence in the environment
The concentrations of LAS have been monitored in several environmental compartments. The monitoring conducted in the Netherlands showed that the concentrations of LAS in the effluent of seven representative municipal sewage treatment plants varied between 0.019 and 0.071 mg/l with an average value of 0.039 mg/l (Matthijs et al. 1999). Concentrations of LAS in sewage sludges have been measured in the range of 2 to 12 g/kg for primary and anaerobically digested sludge (most in the range 4-10 g/kg), whereas aerobically digested sludge and activated sludge contained 2.1-4.3 g/kg and 0.09-0.86 g/kg, respectively (Painter 1992). A monitoring of contaminants in sludge samples from municipal sewage treatment plants in Denmark showed that the concentrations of LAS varied between 0.01 and 16 g/kg (Tørsløv et al. 1997). The median concentration of all examined sludge samples (20) was 0.53 g/kg, whereas the medians were 0.02 g/kg for 11 activated sludge samples and 0.94 g/kg for 9 samples consisting of a mixture of activated and anaerobically digested sludge (Madsen et al. 1998). LAS are found in soils that are treated with sewage sludge as a fertilizer. E.g., the concentration of LAS ranged from 2.5 to 40.3 mg/kg (median 25 mg/kg) in 7 soil samples that were collected immediately after dosing of the fields with sludge; these values fell to ‘control’ values within 21 to 122 days (Painter 1992).
Aquatic sediments may also contain LAS at mg/kg levels. E.g., the LAS concentrations were 1.5 to 10 mg/kg in 10 river sediments in Germany, whereas 25 to 174 mg/kg were found at four other sites. A Danish monitoring of contaminants in coastal marine sediments showed LAS concentrations of up to 22 mg/kg (Lillebæltssamarbejdet 1998). The highest concentration of 22 mg/kg was found in a fjord in the vicinity of the discharge of effluent from a municipal wastewater treatment plant.
3.3.2 Environmental fate
Biodegradation pathways
The initial step in the biodegradation of LAS under aerobic conditions is an w -oxidation of the terminal methyl group of the alkyl chain to form a carboxylic acid. Further degradation proceeds by a stepwise shortening of the alkyl chain by b -oxidation leaving a short-chain sulfophenyl carboxylic acid. In the presence of molecular oxygen the aromatic ring structure hydrolyses to form a dihydroxy-benzene structure which is opened before desulfonation of the formed sulfonated dicarboxylic acid. The final degradation steps have not been investigated in details but are likely to occur by general bacterial metabolic routes involving a total mineralisation and assimilation into biomass (Steber and Berger 1995). Both the initial w -oxidation and the hydroxylation of the ring structure of LAS require molecular oxygen, and they are not expected to take place under anoxic conditions (Steber and Berger 1995).
Aerobic biodegradability
Numerous data on primary and ultimate biodegradation of LAS have been reported. Primary degradation of 93-97% was measured as MBAS removal in OECD tests (Schöberl et al. 1988) and removal in wastewater treatment plants are reported to exceed 85% measured as MBAS (Steber and Berger 1995). The removal of LAS in wastewater treatment plants includes sorption to the sludge particles. For different treatment plants the sorbed amount was reported to be 3-15% with a total removal of 95-99% (Painter 1992). The ultimate biodegradation of LAS in aerobic screening tests fulfils the requirements for ready biodegradability in OECD 301 tests (Table 3.13). The degradation of LAS does not lead to an accumulation of metabolites as indicated by a 95% DOC removal in a test for detection of recalcitrant metabolites (Steber and Berger 1995).
Table 3.13
Ultimate aerobic biodegradability of LAS.
LAS |
Test |
Result |
Reference |
C10-13 |
Closed bottle test, 28 d |
55-65% ThOD |
Schöberl et al. 1988 |
Modified OECD screening test, 28 d |
73-84% DOC |
Schöberl et al. 1988 |
|
CO2 evolution test, 28 d |
45-76% ThCO2 |
Schöberl et al. 1988 |
Anaerobic biodegradability
Ultimate biodegradation of LAS under anoxic conditions has not been documented, and the known mechanisms that precede the aerobic mineralization, i.e. the w -oxidation and the hydroxylation of the benzene ring, require molecular oxygen. A primary anaerobic biodegradation of LAS may occur dependent on the environmental conditions. This has, e.g., been shown in continuous stirred tank reactors with anaerobically digested sludge, operated at 37° C, in which the anaerobic transformation of C12 LAS corresponded to between 20 and 25% of the initial concentration (Angelidaki et al. 2000). Another recent study by Denger and Cook (1999) showed that commercial LAS and C12-3 LAS were desulfonated under sulphur-limited anoxic conditions. The two studies show that LAS can be attacked and transformed by bacteria in the absence of molecular oxygen which implies that LAS is possibly not entirely persistent in anoxic environments. However, it is too early to assess the environmental relevance of the observed anaerobic transformation of LAS, and studies of the fate of LAS in aquatic sediments that are adapted via the continuous discharge of treated effluents should be conducted in the future. Sulphur-limited conditions are not expected to exist in anoxic sediments and, especially, marine sediments usually contain high levels of sulfate. E.g., the molar concentrations of SO4-- in coastal Danish sediments during summer have been measured to approximately 16 mM (Randers Fjord and Kysing Fjord) and 25 mM (Limfjorden) at the surface and approximately 5 mM in deeper layers (Jørgensen 1977; Sørensen et al. 1979).
Fate of LAS in sludgeamended soil
Since LAS are generally not degraded under anoxic conditions, levels of LAS in the g/kg range can be found in sludge which is applied to agricultural soil. The LAS in the sludge will normally biodegrade rapidly in well-aerated and aerobic soils. An extensive environmental monitoring of LAS concentrations in agricultural fields following sludge-amendment showed losses of LAS ł 98% in the majority of the sites with calculated half-lives for LAS in soil between 7 and 22 days (Holt et al. 1989; Waters et al. 1989). The field monitoring data are in agreement with laboratory studies of the mineralization of 14C-labelled C12-LAS in mixtures of sludge and soil. In these studies, more than 68% of the added 14C-LAS was mineralized during 2 months, when aerobic conditions prevailed, while a lower mineralization was seen in mixtures that were partly anoxic (Gejlsbjerg et al., in press). The fate of LAS was recently evaluated for two catchment areas reflecting the eastern and western parts of Denmark (Madsen et al. 1999). The model simulations indicated that worst-case LAS concentrations in the upper 0-15 cm will be between 1 and 10 mg/kg with higher concentrations immediately after sludge application (sludge dosage: 2,000 kg/ha/year; LAS concentration 16 g/kg). The LAS concentrations in sludge are usually much slower than 16 g/kg and, hence, typical LAS concentrations in the upper 0-15 cm were estimated to between 0.1 and 1 mg/kg with higher concentrations immediately after sludge application. A substantial fraction of 98-99% of the sludge-bound LAS was predicted to degrade in the upper soil layer within one year, and the degree of leaching of LAS to depths below 1 m was predicted to be < 1.3% of the LAS applied with sludge (Madsen et al. 1999).
Bioaccumulation
Earlier studies of the bioaccumulation of LAS in aquatic organisms have mainly been performed with 14C- or 35S-radiolabelled LAS. By using radioactivity measurements, whole fish BCF values for C12 LAS have been determined to 108-280 for bluegill sunfish (Lepomis macrochirus; Bishop and Maki 1980), 173-245 for fathead minnow (Pimephales promelas; Kimerle et al. 1975), and 231 for zebra fish (Brachydanio rerio; Coenen 1988). Several studies show that LAS are transformed in fish (Comotto et al. 1979; Kikuchi et al. 1980; Newsome et al. 1995), but the experimental data do not allow a quantitative analysis of intact LAS and biotransformation products. Identification of metabolites suggests that biotransformation of LAS occurs via w -oxidation followed by b -oxidation. These processes lead to the formation of short-chained intermediates that are less toxic because of their lower lipophilicity compared to LAS (Newsome et al. 1995). Bioconcentration experiments using radiolabelled compounds are likely to overestimate the BCF for the intact surfactant because the radiotracer technique does not distinguish between the parent compound and radiolabelled metabolites.
Tolls (1998) examined the uptake and depuration of different LAS homologues by chemical analyses of the parent compound. The BCF tended to increase with increasing alkyl chain length but also the position of the aryl sulfonate moiety was important. A higher BCF was seen for LAS isomers with the aryl sulfonate attached to the second carbon at the alkyl chain, i.e. C11-2, C12-2, and C13-2 (Table 3.14). As it can be seen from the data in Table 3.14, the obtained BCF values differ markedly which indicates that inter-experimental difference exists. The only difference between the experiments with C12-2 LAS is the concentration of the compound in the tested mixture. Apparently, the BCF is inversely related to the concentration of the compound in the mixture, i.e. a higher BCF is obtained with decreasing test concentrations. As the toxicity of LAS is expected to decrease after the primary biotransformation, the BCF studies using chemical analyses of intact surfactant are of higher value than experiments based on radiolabelled compounds. The data in Table 3.14 indicate that the homologues in commercial LAS (i.e., C10-x - C13-x) have a low-to-moderate bioaccumulation potential with the exception of the C13-2 LAS.
Table 3.14
Whole body BCF values in fathead minnow (Pimephales promelas). Data from Tolls (1998).
LAS |
Uptake/depuration period |
BCFss |
C10-2 |
168-192 h/96 h |
6.0 (46) |
C11-2 |
168-192 h/96 h |
31.9 (29) |
C12-2 |
168-192 h/96 h |
99.1 – 211.5 |
C13-2 |
168-192 h/96 h |
987.2 (22) |
C11-5 |
168-192 h/96 h |
6.1 – 9.8 |
C12-5 |
168-192 h/96 h |
10.0 (44) |
C13-5 |
168-192 h/96 h |
34 (34) |
C12-6 |
168-192 h/96 h |
31.9 (48) |
C10-in |
168-192 h/96 h |
3.0 (50) |
C11-in |
168-192 h/96 h |
9.1 (41) |
C12-in |
168-192 h/96 h |
29.9 (27) |
C13-in |
168-192 h/96 h |
112.4 (28) |
Note: Cn-in represents the ‘inner isomers’, i.e.
the sum of the 3-, 4-, 5-, 6-, and 7-sulfophenylalkanes, in contrast to the 2-isomer. The
values in parentheses specify the relative standard variation in %.
3.3.3 Effects on the aquatic environment
Numerous studies have been performed to determine the effects of LAS towards aquatic organisms. The aquatic effect concentrations that were observed in these studies are highly variable. This variation is partly related to the testing of different isomers and homologues, but it may also be due to the specific test conditions and species. The length of the alkyl chain is an important factor determining the aquatic toxicity. In general, the homologues with the highest number of carbons in the alkyl chain are more toxic than are those with shorter alkyl chains. Today, commercial LAS have a homologue distribution between C10 and C13 with a typical average alkyl chain length of C11.6.
Algae
The widest range in the toxicity of LAS towards species belonging to the same group is found for algae (Table 3.15). Approximately 90% of the data found in the literature fall between 0.1 and 100 mg/l. Typical ranges of EC50 values are 1 to 100 mg/l for fresh water species and < 1 to 10 mg/l for marine species (Painter 1992). A very low EC100 value of 0.025 mg/l was determined for Gymnodium breve (Hitchcoch and Martin 1977). Previous studies in which Gymnodium breve was exposed with AES confirm that this species is highly sensitive to surfactants (Kutt and Martin 1974), and occasionally available data for Gymnodium breve should therefore not be used for comparison of the aquatic toxicity between various surfactants.
Table 3.15
Effects of LAS to algae.
Species |
LAS |
EC50 (mg/l) |
Test duration |
Reference |
Selenastrum capricornutum |
C10 |
270 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
C11 |
111 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
C12 |
48 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
C13 |
30 |
72 h |
Verge et al. 1996 |
Selenastrum capricornutum |
C14 |
18 |
72 h |
Verge et al. 1996 |
Navicula pelliculosa |
C13 |
1.4 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
C13 |
5 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
C13 |
116 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
C12 |
0.9 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
C12 |
29 |
96 h |
Lewis and Hamm 1986 |
Dunaliella sp. |
C12 |
3.3 (3.0-3.7)** |
24 h |
Utsunomiya et al. 1997 |
Chlorella pyrenoidosa |
C12 |
29 (38-31)** |
96 h |
Utsunomiya et al. 1997 |
Natural periphyton |
C11.9 |
3.3 |
21 d |
Lewis et al. 1993 |
Natural algae populations |
C13 |
1.9* |
3 h |
Lewis and Hamm 1986 |
Natural algae populations |
C12 |
3.4* |
3 h |
Lewis and Hamm 1986 |
* Effect concentrations based on measured concentrations.
** Parentheses indicate 95% confidence intervals.
Invertebrates
LC50 values have been found in the range of 1 to 10 mg/l when Daphnia magna were exposed with LAS homologues between C10 and C13. The acute toxicity of LAS to Daphnia magna generally increases with increasing alkyl chain length. This is illustrated by studies performed by Maki and Bishop (1979) showing that LAS homologues ł C14 produce EC50 values below 1 mg/l (Table 3.16). Similar results were obtained in a study of LAS homologues between C10 to C14 as the 48 h-LC50 values were 1.2 mg/l for C14 LAS and 53.1 mg/l for C10 LAS (Kimerle and Swisher 1977). A study with the marine crustacean Acartia tonsa indicated that a C10-13 LAS affected the survival at 0.54 mg/l (LC50) and the development rate at 0.51 mg/l (EC50) after 8 days of exposure. The 48 h-LC50 that was obtained in the same study with Acartia tonsa was 2.1 mg/l (Kusk and Petersen 1997). Metabolites from biotransformation of LAS are reported to have a much lower toxicity to invertebrates compared to the toxicity of the intact surfactant (Painter 1992).
Table 3.16
Effects of LAS to Daphnia magna unless otherwise indicated.
LAS |
EC50/LC50 |
Test duration |
Reference |
C18 |
0.12* |
48 h |
Maki and Bishop 1979 |
C16 |
0.11* |
48 h |
Maki and Bishop 1979 |
C14 |
0.68* |
48 h |
Maki and Bishop 1979 |
C13 |
2.6* |
48 h |
Maki and Bishop 1979 |
C13 |
2.19 |
96 h |
Maki 1979 |
C13 |
1.17 |
21 d |
Maki 1979 |
C13 |
1.11 |
21 d |
Maki 1979 |
C12 |
5.9* |
48 h |
Maki and Bishop 1979 |
C11 |
21.2* |
48 h |
Maki and Bishop 1979 |
| C10 | 29.5* (27.9-31.1)** |
48 h | Maki and Bishop 1979 |
| C11.8 | 3.94 (2.87-6.83)** |
96 h | Maki 1979 |
| C11.8 | 1.67 (1.228-2.18)** |
21 d | Maki 1979 |
| C11.8 | 1.50 (0.75-3.33)** |
21 d (reproduction) |
Maki 1979 |
| C10-13 | 0.54 Acartia tonsa |
8 d (survival) |
Kusk and Petersen 1997 |
| C10-13 | 2.1 Acartia tonsa |
48 h (survival) |
Kusk and Petersen 1997 |
* Effect concentrations based on measured concentrations.
** 95% confidence intervals.
Fish
The toxicity of LAS to fish generally increases with increasing alkyl chain length, and approximately a 10-fold difference in toxicity between homologues separated by two carbon atoms has been observed. As also noted for invertebrates, fish are less susceptible to metabolites from biotransformation of LAS (Painter 1992). LC50 values below 1 mg/l were found for C11.9 (0.71 mg/l), C13 and C14 (both 0.4 mg/l) in studies with fathead minnow (Table 3.17) and for C10-15 (0.36 mg/l; 96 h) in a study with rainbow trout (Brown et al. 1978).
Table 3.17
Effects of LAS to fathead minnow (Pimephales promelas) unless otherwise indicated.
LAS |
LC50 (mg/l) |
Test duration |
Reference |
C11.9 |
0.71* (0.49-0.98)** |
7 d |
Fairchild et al. 1993 |
C14 |
0.5 LOEC:0.05-0.10 (estimated) |
96 h |
Macek and Slight 1977 |
C13 |
1.8 LOEC: 0.12-0.28 |
96 h |
Macek and Slight 1977 |
C12 |
6.6 LOEC: 1.08-2.45 |
96 h |
Macek and Slight 1977 |
C11 |
21.9 LOEC: 7.2-14.5 |
96 h |
Macek and Slight 1977 |
C10 |
57.5 LOEC:14-28 (estimated) |
96 h |
Macek and Slight 1977 |
C10-13 |
4.6 LOEC:1.02-2.05 (estimated) |
96 h |
Macek and Slight 1977 |
C10 |
43 |
48 h |
Kimerle and Swisher 1977 |
C11 |
16 |
48 h |
Kimerle and Swisher 1977 |
C12 |
4.7 |
48 h |
Kimerle and Swisher 1977 |
C13 |
0.4 |
48 h |
Kimerle and Swisher 1977 |
C14 |
0.4 |
48 h |
Kimerle and Swisher 1977 |
C10-15 |
0.36* (0.25-0.51)** |
96 h |
Brown et al. 1978 |
C13 |
NOEC:0.15* |
30 d |
Maki 1979 |
C11.8 |
NOEC:0.9* |
30 d |
Maki 1979 |
C11.2 |
LOEC:5.1-8.4* (life cycle) |
- |
Holman and Macek 1980 |
C11.7 |
LOEC:0.48-0.49* (life cycle) |
- |
Holman and Macek 1980 |
C13.3 |
LOEC:0.11-0.25* (life cycle) |
- |
Holman and Macek 1980 |
* Effect concentrations based on measured concentrations.
** 95% confidence intervals.
Sediment organisms
LAS sorb to sediment with partition coefficients of 50 to 1,000. The toxicity of LAS bound to sediment is relatively low compared to LAS in solution. NOEC and LOEC values were as high as 319 and 993 mg LAS/kg, respectively, for the sediment-living Chironomus riparius. The corresponding NOEC for LAS in solution was as low as 2.4 mg/l indicating that only a small fraction of the sorbed LAS was bioavailable (Painter 1992). Bressan et al. (1989) investigated the effects of LAS dissolved in water and found acute effects in the range of 0.25 to 200 mg/l dependent of the species. Copepods and embryos of the sea urchin Paracentrotus lividus were the most sensitive organisms. LAS dissolved in water may also cause chronic effects like reduction of the growth rate of the marine mussel Mytilus galloprovincialis. LAS sorbed to sediments did not have similar effects. No alterations of the treated organisms were observed although the LAS concentrations in the sediment were 3 to 10 times higher than the effect concentrations observed for LAS in water. The 96 h-LC50 values for sediment-bound LAS were 182.5 mg/kg and 200 mg/kg for the bivalve molluscs Unio elongatulus and Anodonata cygnea.
3.3.4 Effects on human healthToxicokinetics and acute toxicity
LAS are readily absorbed by the gastrointestinal tract after oral administration in animals. LAS are not readily absorbed through the skin (IPCS 1996). The bulk is metabolized in the liver to sulfophenylic carboxyl acids. The metabolites are excreted primarily via the urine and faeces. The main urinary metabolites in rats are sulfophenyl butanoic acid and sulfophenyl pentanoic acid. Accumulation of LAS or its main metabolites has not been established in any organ after repeated oral ingestion (SFT 1991).
Dodecylbenzene sulfonate (C12 LAS) was administrated daily in the diet at a dose level of 1.4 mg/kg body weight for 5 weeks. Of the administered dose of C12 LAS, 52.4% was excreted in faeces and 29.4% in urine during the dosing period. A single application of 0.385 mg C12 LAS per rat resulted in a total elimination of 85% within the first 24 hours and 95% within 10 days (Lay et al. 1983). No data on skin absorption were identified, but the skin absorption of anionic surfactants is generally considered to be very low.
Table 3.18
Acute toxicity (LD50) of alkyl benzene sulfonates.
Surfactant |
Species |
Route |
LD50 (mg/kg/ body weight) |
Reference |
Branched alkylbenzene sulfonate |
Rat |
Oral |
700 – 2,480 |
SFT 1991; Gloxhuber and Künstler 1992 |
LAS |
Rat |
Oral |
401 – 1,900 |
IPCS 1996 |
LAS |
Mouse |
Oral |
1,259 – 2,300 |
IPCS 1996 |
LAS |
Rabbit |
Dermal |
> 500 |
CIRP 1993 |
No serious injuries or fatalities in man have been reported following accidental ingestion
of LAS-containing detergent (Painter 1992; IPCS 1996). The main clinical signs observed
after oral administration to rats of doses near or greater than the LD50 values consisted
of reduced voluntary activity, diarrhoea, weakness etc. Death usually occurred within 24
hours of administration. Rats appear to be more sensitive to LAS than mice (IPCS 1996).
Skin and eye irritation
LAS and branched alkylbenzene sulfonates may cause irritation of the eyes, skin and mucous membranes. LAS are relatively more irritating to the skin than the corresponding branched alkylbenzene sulfonates (KEMI 1990). The potential of LAS to irritate the skin depends on the concentration applied. LAS have been classified as irritating to skin at concentrations above 20% according to EU-criteria. Human skin can tolerate contact with solution of up to 1% LAS for 24 hours resulting in only mild irritation (IPCS 1996). Application of > 5% LAS to the eyes of rabbits produced irritation. Concentration of < 0.1% LAS produced mild to no irritation (CIRP 1993).
Sensitization
Skin sensitization was not seen in 2,294 volunteers exposed to LAS or in 17,887 exposed to formulations of LAS (Nusair et al. 1988).
Subchronic and long-term toxicity
A feeding study indicated that LAS, when administered for 2 years at extremely high levels (0.5%) in the diets to rats, produced no adverse effects on growth, health or feed efficiency (Buehler et al. 1971).
Mutagenicity and carcinogenicity
The mutagenic potential of LAS was tested using Salmonella typhimurium strains, using Ames test. In these studies, LAS was not mutagenic (Inoue and Sunakawa 1980). The available long-term studies are inadequate for evaluating the carcinogenic potential of LAS in laboratory animals. The studies available (oral administration to rats and mice) do not show any evidence of carcinogenicity (Gloxhuber and Künstler 1992; IPCS 1996).
Reproductive toxicity
LAS was applied daily from day 0 through day 20 of gestation to the shaved skin of pregnant rats. The applied concentrations of LAS were 0.05-0.5%, and the doses remained on the skin. Furthermore, concentrations of 1%, 5% and 20% LAS were applied to the skin of pregnant rats, and these doses were removed 30 minutes after exposure. The only effects attributed to LAS were reduced body weight in the dams given the highest concentration (20%), and local skin changes in the dams which received the two highest concentrations (5% and 20%). There were no findings indicative of effects of LAS on the foetal parameters evaluated and no indication of teratogenic or embryotoxic effects (Daly and Schroeder 1980).
A 20% LAS solution (0.1 ml) was applied twice daily to the dorsal skin (2 x 3 cm) of pregnant 1CR/Jc1 mice during the preimplantation period (days 0-3 of gestation). A significant number of embryos collected on day 3 were severely deformed or remained at the morula stage. Nomura et al. (1980, 1987) reported that the number of embryos in the oviducts was significantly greater for the mice dosed with LAS as compared to the control mice used in that study. No pathological changes were detected in the major organs of the dams.
In general no specific effect of LAS on reproductive processes has been seen, although dosages causing maternal toxicity may also induce some effects on reproduction. No teratogenic effects attributed to LAS exposure were observed (Gloxhuber and Künstler 1992; IPCS 1996).
Classification
LAS are classified as Irritant (Xi) with the risk phrases R38 (Irritating to skin) and R41 (Risk of serious damage to eyes) according to CESIO (CESIO 2000).
LAS are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
3.4 Secondary alkane sulfonates
Alkane sulfonates are used in liquid detergents like, e.g., dishwashing agents, cleaning agents, and hair shampoos, frequently in combination with AES. Commercial products are almost exclusively composed of secondary alkane sulfonates (SAS) with the following structure:
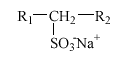
The alkyl chain (R1+R2), normally C11 to C17, is linear and the SO3- group is placed randomly along the alkyl chain. Thus, e.g., C14 alkane sulfonate is a mixture of the six isomers 2-, 3-, 4-, 5-, 6- and 7-sulfotetradecane.
No data were found on the occurrence of SAS in the environment.
3.4.1 Environmental fate
Biodegradation pathways
The of SAS biodegradation has only been scarcely investigated. A pathway similar to that of LAS involving an initial w /b -oxidation is an obvious assumption, but this has not yet been proven. One study suggests that the initial degradation step is a desulfonation requiring molecular oxygen (Painter 1992). This process involves the formation of a hydroxysulfonate which is hydrolysed to inorganic sulfate and a ketone. The ketone is subsequently oxidised to an ester which is cleaved to acetic acid and the corresponding alcohol (Painter 1992).
Aerobic biodegradability
SAS undergo rapid primary biodegradation with MBAS removals higher than 90% within a few days (Swisher 1987). Removals of 96% were seen in the OECD screening test for primary biodegradation (Schöberl et al. 1988). In activated sludge simulation tests, 96% of C10-18 SAS was removed, while the parent C13-18 SAS was removed by 83-96% (Painter 1992). The fate of a 14C-labelled C17 SAS was followed in a continuous activated sludge test to illustrate the ultimate biodegradation. After 3 days, 47% of the added C17 SAS were detected as 14CO2 and 25% were incorporated into sludge biomass (Steber and Berger 1995). The ultimate biodegradability of SAS fulfils the criteria for ready biodegradability in OECD 301 tests (Table 3.19).
Table 3.19
Ultimate aerobic biodegradability of SAS.
Compound |
Test |
Result |
Reference |
C12-18 |
Closed bottle test, 30 d |
93% ThOD |
Painter 1992 |
C13-17 |
Closed bottle test, 28 d |
99% ThOD |
Madsen et al. 1994 |
C13-18 |
Closed bottle test, 28 d |
63-95% ThOD |
Schöberl et al. 1988 |
Modified OECD screening test, 28 d |
88-96% DOC |
Schöberl et al. 1988 |
|
CO2 evolution test, 28 d |
56-91% ThCO2 |
Schöberl et al. 1988 |
Anaerobic biodegradability
Wagener and Schink (1987) investigated the anaerobic biodegradability of SAS in tests incubated with either digested sludge or creek sludge and came to the conclusion that alkyl sulfonates are not degraded under anoxic conditions.
Bioaccumulation
No experimental data describing the bioaccumulation potential of SAS were found in the literature.
3.4.2 Effects on the aquatic environment
Algae
The toxicity of various SAS homologues was determined in tests with Chlamydomonas variabilis. After 24 hours of exposure at 20° C, there was a tendency to an increased toxicity with increasing chain length. The EC50 values were 125 mg/l for C10.3, 74.9 mg/l for C11.2, 32.4 mg/l for C14, 15.8 mg/l for C15, 9.42 mg/l for C16, 3.93 mg/l for C17, 3.71 mg/l for C18.9, and 8.47 mg/l for C20.7 (Lundahl and Carbridenc 1978).
Invertebrates
The same tendency to an increased toxicity of SAS with increasing chain length was seen in tests with Daphnia magna. The tests with Daphnia magna showed 24 h-EC50 values at 319 mg/l for C10.3, 133 mg/l for C11.2, 111 mg/l for C14, 34.2 mg/l for C15, 30.1 mg/l for C16, 12.3 mg/l for C17, 3.31 mg/l for C18.9, and 6.30 mg/l for C20.7 (Lundahl and Carbridenc 1978). Schöberl et al. (1988) reported an EC50 range of 8.7-13.5 mg/l for daphnia in studies with C13-18 SAS, whereas Painter (1992) reported a lower EC50 range of 0.7-6 mg/l for C15-18 SAS.
Fish
Also for fish the longer chain length SAS are more toxic than the shorter chained homologues (Table 3.20). This has been shown both for minnow (Lundahl and Carbridenc 1978) and for bluegill sunfish (Painter 1992). Schöberl et al. (1988) reported a range of LC50 values of 3-24 mg/l for C13-18 SAS in tests with fish species that were not specified.
Table 3.20
Effects of SAS to fish.
Species |
SAS |
LC50 |
Test duration |
Reference |
Minnow (Phoxinus phoxinus) |
C14 |
34.5 |
24 h |
Lundahl and Carbridenc 1978 |
Minnow |
C15 |
8.5 |
24 h |
Lundahl and Carbridenc 1978 |
Minnow |
C16 |
3.11 |
24 h |
Lundahl and Carbridenc 1978 |
Bluegill sunfish (Lepomis macrochirus) |
C13 |
144 |
96 h |
Painter 1992 |
Bluegill sunfish |
C16 |
4.6 |
96 h |
Painter 1992 |
Bluegill sunfish |
C18 |
1.3 |
96 h |
Painter 1992 |
Fish |
C13-18 |
3-24 |
- |
Schöberl et al. 1988 |
3.4.3 Effects on human health
Toxicokinetics and acute toxicity
SAS are readily absorbed from the gastrointestinal tract of rats after oral administration. Following administration of C12 and C16 SAS the main metabolite is butyric acid-4-sulfonate. This metabolite is eliminated in the urine (McDermott et al. 1975; Taylor et al. 1978).
The acute toxicity of a SAS of non-specified chain length in the rat was moderate with LD50 values between 1,000 and 3,000 mg/kg body weight when administrated by the oral route (Falbe 1986; SFT 1991; Gloxhuber and Künstler 1992).
Skin and eye irritation
The irritating potential of SAS to skin is almost the same as that of alkyl sulfates. Concentrations of more than 20% alkane sulfonate are strongly irritating to the skin of rabbits (SFT 1991).
Chronic toxicity, carcinogenicity, mutagenicity
Subchronic studies with rats receiving 300 mg SAS/kg body weight/day orally for 45 and 90 days revealed no adverse effects. Similarly, rats fed 0.5% SAS in their diets for 91 days developed no adverse symptoms (Scailteur et al. 1986; Painter 1992). There was no indication of increased risk of cancer after oral ingestion of SAS in studies that were not further specified (Falbe 1986; SFT 1991).
Classification
The skin irritating potential of SAS is about the same as for alkyl sulfates. SAS may therefore also be classified as Irritant (Xi) with R38 (Irritating to skin) and R41 (Risk of serious damage to eyes).
SAS are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
3.5 a -Olefine sulfonates (AOS)
a -Olefine sulfonates (AOS) are used in laundry powder detergents, liquid dishwashing agents, as well as in hair shampoos, and mainly in Japan and the USA (Steber and Berger 1995). AOS consist of a mixture of alkene sulfonates (about 60-65%) and hydroxyalkane sulfonates (about 30-40%). The normally linear C-chain in alkene 1-sulfonates and hydroxyalkane 1-sulfonates may contain 11 to 20 carbons with 14 to 18 carbons as the usual range (Painter 1992).
The alkene sulfonates have the structure:
H3C-(CH2)m-CH=CH-(CH2)n-SO3-Na+
(m = 1, 2, 3, …; n = 0, 1, 2, …; m + n = 9-15)
The hydroxyalkane sulfonates have the structure:
R-CH2-CH(OH)-(CH2)m-SO3-Na+
(R = C7-13; m = 1,2,3)
The a -olefine sulfonates are expressed as, e.g., C18 AOS or Cx AOS if the number of C atoms is not known. The hydroxyalkane sulfonates are expressed as C18-xOH AOS, where x indicates the C atom at which the –OH group is attached on the carbon chain.
No data were found on the occurrence of AOS in the environment.
3.5.1 Environmental fateBiodegradation pathways
Very little is known about the biodegradation pathways of AOS. Steber and Berger (1995) report a hypothetical pathway involving an initial desulfonation catalyzed by an alkane sulfonate-a -hydroxylase yielding a desulfonated ketene that could be hydrolysed to the corresponding acid.
Aerobic biodegradability
AOS are rapidly primary biodegradable with MBAS removals between 95 and 100% in 2 to 8 days in river water and inoculated media (Painter 1992). The ultimate biodegradability of AOS exceeds the pass requirements in OECD 301 tests for ready biodegradability. Schöberl et al. (1988) report 85% DOC removal in the modified OECD screening test, 85% ThOD in the closed bottle test, and 65-80% ThCO2 in the Sturm test. In activated sludge simulation tests, AOS was removed by 100% MBAS and 88% DOC (Painter 1992). The alkene sulfonates and hydroxyalkane sulfonates in commercial AOS are both ultimately biodegraded as approximately 84% ThCO2 was obtained during degradation of C14, C16, and C18 within 27 days, whereas the corresponding 3-hydroxyalkane sulfonates were degraded by approximately 86% under the same conditions (Painter 1992).
Anaerobic biodegradability
The studies of Wagener and Schink (1987) indicate that AOS are not degraded anaerobically. However, Painter (1992) reports a range of 31% to 43% MBAS removal under anoxic conditions indicating primary biodegradation.
Bioaccumulation
No experimental data describing the bioaccumulation potential of AOS were found in the literature.
3.5.2 Effects on the aquatic environment
Toxicity studies describing the effects of AOS to aquatic organisms have mainly been performed with fish. Only a few data have been found describing the effects towards algae and crustaceans.
Algae
Schöberl et al. (1988) report a range of 10-100 mg/l for C14-18 AOS as being toxic to the growth of algae.
Invertebrates
EC50 values for Daphnia magna have been determined within the range 5-50 mg/l for C14-18 AOS (Schöberl et al. 1988). Another study with Daphnia magna, referred by Painter (1992), showed EC50 values of 16.6 mg/l for C14-16 AOS and 7.7 mg/l for C16-18 AOS.
Fish
The studies performed with fish show that the higher homologues of AOS are more toxic than the lower ones. This has been illustrated for different fish species (see Table 3.21).
Table 3.21
Effects of AOS to fish.
Species |
AOS |
LC50 (mg/l) |
Test duration |
Reference |
Harlequin fish (Rasboa heteromorpha) |
C14-16 C16-18 |
3.3 0.5 |
96 h 96 h |
Reiff et al. 1979 |
Brown trout (Salmo trutta) |
C14-16 C16-18 |
2.5-5 0.5 |
96 h 96 h |
Reiff et al. 1979 |
Golden orfe (Idus idus) |
C14-16 C16-18 |
3.4 0.9 |
96 h 96 h |
Reiff et al. 1979 |
Fathead minnow (Pimephales promelas) |
C14-16 C16-18 |
5.3 1.4 |
24 h 24 h |
Painter 1992 |
Rainbow trout (Salmo gairdneri) |
C14-16 C16-18 |
5.1 0.8 |
24 h 24 h |
Painter 1992 |
3.5.3 Effects on human health
Toxicokinetics and acute toxicity
The absorption of AOS through intact skin is considered to be very low (IPCS 1996). sUnchanged a -olefine sulfonate (AOS) and/or metabolites of AOS are primarily eliminated in the urine and, to a lesser extent, in the faeces within 24 hours of administration. The chemical structures of the metabolites have not yet been identified.
AOS has a moderately low acute oral toxicity as indicated by LD50 values between 1,300 and 2,400 mg/kg body weight for rats and between 2,500 and 4,300 mg/kg body weight for mice (SFT 1991; IPCS 1996). The toxic effects at high oral doses were reduced voluntary activity, diarrhoea and anaemia (IPCS 1996).
Skin and eye irritation
AOS are mildly to moderately irritating to human skin depending on the concentration. In patch tests, human skin can tolerate contact to solutions containing up to 1% AOS for 24 hours resulting in only mild irritation (IPCS 1996). Instillation in the rabbit eye of 0.5% AOS caused no irritation after 24 hours, while 1% AOS caused a weak irritation (Gloxhuber 1974).
Chronic toxicity, carcinogenicity, mutagenicity
The long-term toxicity and potential tumorigenic activity of AOS were assessed in a 2 year feeding study in rats at dietary levels of 0.1, 0.25 and 0.5%. No adverse clinical effects were observed, and survival rates were not affected by treatment with AOS. Histological examination of the tissues did not provide any evidence of toxicity or tumour induction (Hunter and Benson 1976). In the Salmonella/microsome assay (Ames test) AOS were tested as negative showing a negligible potential to cause genetic damage (Yam et al. 1984).
Reproductive toxicity
AOS were studied in rabbits, mice and rats for teratogenic potential. AOS were administered orally once a day by gavage on day 6-15 of pregnancy in mice and rats and on day 6-18 of pregnancy in rabbits. The doses were from 0.2–600 mg/kg body weight. The study showed no evidence of teratogenic potential (Palmer 1975b).
Classification
AOS are classified as Irritant (Xi) with the risk phrases R38 and R41 for concentrations > 80% and R36/38 (Irritating to eyes and skin) for concentrations of 40-80% according to CESIO (CESIO 2000).
AOS are not included in Annex 1 of the list of dangerous substances of Council Directive 67/548/EEC.
3.6 Sulfosuccinates
Sulfosuccinates are used in special detergent formulations and personal care products. Besides, sulfosuccinates are used as emulsifiers in the textile, plastics, photography and leather industries (Hales 1993; Steber and Berger 1995). The structurally related alkyl ether sulfosuccinates are used in personal care products. Sulfosuccinates have the following structure:
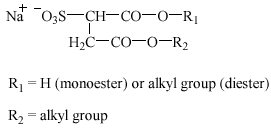
The alkyl chain(s) normally consist of less than nine carbons and can be either linear or branched. Branching increases the water solubility.
No data were found on the occurrence of sulfosuccinates in the environment.
3.6.1 Environmental fate
Biodegradation pathways
Relatively few studies have attempted to elucidate the biodegradation pathway of sulfosuccinates. High removals of carbon but no release of inorganic sulfate suggest that the biodegradation is initiated by hydrolysis of the ester bonds followed by b -oxidation of the alcohols (Steber and Berger 1995). Hales (1993) studied the formation of metabolites during degradation of C6-8 dialkyl sulfosuccinate under aerobic and anoxic conditions. This study confirmed the ester cleavage leaving one of two structural distinct monoalkyl sulfosuccinates, one being readily degraded and the other being less readily degraded. The suggested pathway for the easily degradable metabolite is hydrolytic cleavage leaving the corresponding alcohol and sulfosuccinate, whereas the other compound is sequentially degraded by w - and b -oxidations. In the absence of molecular oxygen, the ester bonds may be cleaved and followed by b -oxidation of the alcohol but the cleavage of the C-S-bond occurs only in the presence of oxygen. Thus a primary biodegradation is possible, whereas ultimate biodegradation is unlikely to occur under anoxic conditions (Hales 1993; Steber and Berger 1995).
Aerobic biodegradability
Data for the aerobic biodegradability have only been found for dialkyl sulfosuccinates and not for the ethoxylated compounds. High degrees of primary biodegradation (97-99%) are reported for C6-8 dialkyl compounds in OECD tests (Schöberl et al. 1988; Hales 1993). The biodegradation is highly affected by the structure of the carbon chain as indicated by a decreased primary biodegradation rate for structures with branched alkyl chains (Steber and Berger 1995). Dialkyl sulfosuccinates are not readily biodegradable according to OECD criteria for ready biodegradability (Table 3.22). Also coupled units tests have shown incomplete biodegradation with 70% DOC removal for C6-8 dialkyl sulfosuccinate (Hales 1993) and 49% for C8 dialkyl sulfosuccinate (Schöberl et al. 1988). A modified semi-continuous activated sludge test for ultimate inherent biodegradability showed 85-94% removal based on measurements of C6-8 dialkyl sulfosuccinate carbon (Hales 1993).
Table 3.22
Ultimate aerobic biodegradability of sulfosuccinates.
Compound |
Test |
Result |
Reference |
C6-8 dialkyl sulfosuccinate |
Modified OECD screening test, 28 d |
51-62% DOC 45-55% ThCO2 |
Hales 1993 |
C8 dialkyl sulfosuccinate |
Closed bottle test, 28 d |
50% ThOD |
Schöberl et al. 1988 |
Anaerobic biodegradability
No data have been found confirming an ultimate biodegradation of sulfosuccinates under anoxic conditions. As described in relation to the biodegradation pathway, only a primary biodegradation is anticipated in the absence of molecular oxygen (Hales 1993; Steber and Berger 1995).
Bioaccumulation
No experimental data describing the bioaccumulation potential of sulfosuccinates were found in the literature.
3.6.2 Effects on the aquatic environment
Very few data describing the aquatic toxicity of sulfosuccinates are available. Schöberl et al. (1988) report EC/LC50 values of 33 mg/l for daphnia and 39 mg/l for fish for C8 dialkyl sulfosuccinate.
3.6.3 Effects on human health
No data are available on the effects on human health. Sulfosuccinates are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
3.7 Fatty acid soaps
Fatty acid soaps are the alkali salts of fatty acids. Soaps are primarily used in toilet soap bars and, also in solid form, as a cleaning agent. Typical raw materials for the production of soap are palm kernel oil (C8-14), coconut oil (C12-16), palm oil (C14-18), and tallow fat (C16-18).
R-CH2-COO-Na+
(R = 10-16)
3.7.1 Occurrence in the environment
Only few data on the concentration of soap in the environment have been found. The monitoring conducted in the Netherlands showed that the concentrations of soap in the effluent of six representative municipal sewage treatment plants varied between 0.091 and 0.365 mg/l with an average value of 0.174 mg/l (Matthijs et al. 1999).
3.7.2 Environmental fate
Biodegradation pathways
The degradation of fatty acids proceeds by b -oxidation in which coenzyme A is involved. Stepwise shortening of the alkyl chain occurs under the formation of acetyl coenzyme A fragments, which are used in living cells for energy production (Steber and Berger 1995). The b -oxidation may proceed in the absence of oxygen as well which implies that the same biodegradation pathway is anticipated in anoxic environments (Steber and Berger 1995).
Aerobic biodegradability
The general method for measuring primary biodegradation of anionics (MBAS analyses) is not applicable for fatty acids and, hence, no concrete data on primary biodegradability of soaps are available (Steber and Berger 1995). However, fatty acid soaps are rapidly and ultimately biodegradable which indicates a rapid primary biodegradation of these compounds. Fatty acids and soaps are ultimately biodegraded in the OECD 301 tests for ready biodegradability as illustrated by the data in Table 3.23.
Table 3.23
Ultimate aerobic biodegradability of fatty acids and soaps.
Compound |
Test |
Result |
Reference |
Na-soap |
Sturm test, 28 d |
80-90% ThCO2 |
Schöberl et al. 1988 |
Ca-stearate |
Sturm test, 28 d |
63% ThCO2 |
Schöberl et al. 1988 |
Na-laurate |
BOD/COD, 10 d |
100% |
Steber and Berger 1995 |
Na-palm kernelate |
BOD/COD, 10 d |
ł 90% |
Steber and Berger 1995 |
Na-oleate |
BOD/COD, 10 d |
100% |
Steber and Berger 1995 |
Na-tallow soap |
BOD/COD, 10 d |
100% |
Steber and Berger 1995 |
Na-stearate |
BOD/COD, 10 d |
> 85% |
Steber and Berger 1995 |
Na-behenate |
BOD/COD, 10 d |
> 75% |
Steber and Berger 1995 |
C8-18 fatty acids |
BOD/COD, 28 d* |
100% |
Steber and Berger 1995 |
C16 fatty acid |
BOD/COD, 28 d* |
100% |
Steber and Berger 1995 |
C18 fatty acid |
BOD/COD, 28 d* |
79% |
Steber and Berger 1995 |
C22 fatty acid |
BOD/COD, 28 d* |
69% |
Steber and Berger 1995 |
*Modified for poorly water-soluble compounds
Anaerobic biodegradability
The anaerobic biodegradability of palmitic acid has been confirmed in a digester model system (Steber and Wierich 1987) and in the more stringent ECETOC/ISO 11734 test (Table 3.24). Gas production measurements in a fermentor, in which the soaps were added in a semi-continuous mode, showed that the anaerobic biodegradability corresponded to 95% degradation of laurate (C12), 70% of oleate and palm kernel-based soap (C18 and C12-18), 60% of tallow-based soap, and only 14% of behenate (C22) (Steber and Berger 1995). Madsen et al. (1996a) examined the anaerobic biodegradability of Na-cocoate (C8-18) in screening tests by using either digested sludge, freshwater swamp material, or marine sediment as inoculum. The biodegradability observed after 28 and 56 days of incubation at 35° C was, respectively, 70 and 93% ThGP in the digested sludge, 60 and 84% ThGP in the freshwater swamp, and 50 and 96% ThGP in the marine sediment.
Table 3.24
Ultimate anaerobic biodegradability of fatty acids and soaps in digested sludge.
Compound |
Type of test and duration |
Result |
Reference |
Palmitic acid, C16 |
Measurement of 14CH4 and 14CO2 evolution, 28 d |
92-97% ThCH4 + ThCO2 |
Steber and Wierich 1987 |
Na-cocoate, |
Measurement of gas production, 35° C, 56 d |
93% ThGP |
Madsen et al. 1996a |
K-cocoate, C12-16 |
Measurement of gas production, 35° C, 56 d |
99% ThGP |
This study (Appendix; Table A8, Figure A8) |
Palmitate, C16 |
Measurement of gas production, 35° C, 28 d |
79-94% ThGP |
Birch et al. 1989 |
Bioaccumulation
No experimental data describing the bioaccumulation potential of fatty acid soaps were found in the literature.
3.7.3 Effects on the aquatic environment
The aquatic toxicity of fatty acid soaps is very variable and seems to be highly dependent on both the species and the specific fatty acid soap tested.
Algae
Schöberl et al. (1988) reported that the growth of algae was inhibited at concentrations of 10-50 mg/l of Ca-soap. Yamane et al. (1984) investigated the effects of C8-18 soap towards three different alga species and obtained EC50 (72 h) values of 10-50 mg/l for Selenastrum capricornutum, 20-50 mg/l for Microcystis aeruginosa, and 10-20 mg/l for Nitzschia fonticula. All of these EC50 were determined by using the growth rate of the algae (Table 3.25).
Table 3.25
Effects of fatty acids and soaps to algae.
Species |
Fatty acid soap |
EC50 |
Test duration |
Reference |
Selenastrum capricornutum |
C8-18 soap |
10-50 |
72 h |
Yamane et al. 1984 |
|
20-50 |
72 h |
|
|
|
10-20 |
72 h |
|
|
Algae |
Ca soap |
10-50 |
Not indicated |
Schöberl et al. 1988 |
Microcystis aeruginosa |
Soap |
18-32 |
96 h |
Canton and Slooff 1982 |
|
180-320 |
96 h |
|
|
Scenedesmus
|
Na-laurate |
53 |
72 h |
BKH |
Na-oleate |
58 |
72 h |
Consulting |
|
Na-palmoil soap |
140 |
72 h |
Engineers 1994 |
|
Na-tallow acid |
190 |
72 h |
|
|
Na-behenate |
230 |
72 h |
|
Invertebrates
The variability in the toxicity of fatty acid soaps towards Daphnia magna is approximately a factor of 20. The effect concentrations reported for Daphnia magna and Gammarus pulex are presented in Table 3.26.
Table 3.26
Effects of fatty acids and soaps to crustaceans.
Species |
Fatty acid soap |
EC50/LC50 |
Test duration |
Reference |
Daphnia magna
|
Soap |
32-56 |
48 h |
Canton and Slooff 1982
|
|
36;NOEC:10 |
21 d (mortality) |
||
|
> 10; NOEC:10 |
21 d (reprod.) |
||
Daphnia magna
|
Na-oleate |
4.2 |
24 h |
BKH Consulting engineers 1994
|
Soap |
10 |
- |
||
Palmoil soap |
25 |
24 h |
||
Talgseife |
40 |
24 h |
||
Haushaltseife |
42.3 |
- |
||
Na-laurate |
48 |
24 h |
||
Lauric acid |
2-5.4 |
48 h |
||
Gammarus |
Hardened tallow soap |
88 |
72 h |
BKH Consulting engineers 1994 |
Fish
Schöberl et al. (1988) reported that the adverse effects of fatty acids to fish depend on the hardness of the water. At a water hardness of 0° dH the LC50 of soap towards golden orfe (Idus idus melanotus) was 6.7 mg/l, while it was 20-150 mg/l at 3-23° dH. The same dependence of water hardness was documented by Kikuchi et al. (1976) who exposed killifish (Oryzias latipes) to Na-soap. In distilled water, the 48 h-LC50 was 5.9 mg/l, while no effects were seen at 84 mg/l, when the water hardness was 25 mg CaCO3/l. A relatively high toxicity has been found for oleic acid as the LC50 was between 0.1-2.1 mg/l for rainbow trout (BKH Consulting Engineers 1994). LC50 values for different fish species are presented in Table 3.27.
Table 3.27
Effects of fatty acids and soaps to fish.
Species |
Fatty acid soap |
LC50 (mg/l) |
Test duration |
Reference |
Golden orfe |
Ca-soap |
6.7 (0° dH) |
Not indicated |
Schöberl et al. 1988 |
Killifish |
Na-soap |
5.9 (distilled water) |
48 h |
Kikuchi et al. 1976 |
Guppy |
Soap |
320-560 (200mgCaCO3/l) |
96 h |
Canton and Sloof 1982 |
Oryzias latipes |
Soap |
1,000-1,800 (200mgCaCO3/l) |
96 h |
Canton and Sloof 1982 |
Rainbow trout |
Oleic acid |
0.1-2.1 |
96 h |
BKH Consulting engineers 1994 |
Rice fish
|
Sodium laurate (C12) |
11 |
96 h |
BKH Consulting engineers 1994 |
Sodium myristate (C14) |
118 |
96 h |
|
|
Sodium stearate (C18) |
125 |
96 h |
|
|
Sodium palmitate (C16) |
150 |
96 h |
|
|
Sodium oleate (C18) |
217 |
96 h |
|
|
Haushaltseife |
1,342 |
96 h |
|
|
Fathead minnow |
Oleic acid |
205 |
96 h |
BKH Consulting engineers 1994 |
Bluegill sunfish |
Lauric acid Oleic acid |
63.3 66.6 |
96 h 96 h |
BKH Consulting engineers 1994 |
Trout (Oncorhynchus kisuth) |
Oleic acid Palmitoleic acid |
12 12 |
33 h 2.5 h |
BKH Consulting engineers 1994 |
Golden orfe (Leuciscus idus melanotus) |
Na-fatty acid soap |
54 |
48 h |
BKH Consulting engineers 1994 |
3.7.4 Effects on human health
Toxicokinetics and acuate toxicity
The rate of percutaneous absorption of sodium laurate is greater than that of most other anionic surfactants. The greatest skin penetration of the human epidermis was found with C10 and C12 soaps (Prottey and Ferguson 1975).
The LD50 –values by oral administration of soaps are more than 10,000 mg/kg body weight for rats. This indicates a very low acute toxicity (Gloxhuber and Künstler 1992).
Skin and eye irritation
The existence of unsaturated carbon chains and carbon chain lengths of C16 to C18 contribute to a low skin irritation effect while soaps based on unsaturated C12-chains may be irritating to the skin (KEMI 1990).
Series of sodium soaps were studied to investigate the effect of the lipophilic chain length upon extraction of amino acids and proteins from the stratum corneum. The soaps, sodium laurate (C12) and sodium myristate (C14) removed most amino acids and proteins from the skin (Prottey and Ferguson 1975).
Soap concentrations of 10% or more may be irritating to skin and concentrations above 30% cause severe local irritation (Gloxhuber and Künsler 1992).
The only soaps that lead to permanent corneal damage are those containing large amounts free alkali and having a pH value of more than 12 (Grant and Schuman 1993). Accidental contact of the human eye with soap or soap powder followed by rapid rinsing of the eyes is not expected to cause severe reactions and reactions observed usually disappear quickly (Gloxhuber and Künsler 1992).
Carcinogenicity
Both oral administration and dermal exposures to soap (potassium soap) gave negative results in carcinogenicity tests with laboratory animals (Gloxhuber and Künsler 1992). Sodium oleate (C18) was given to rats in concentrations of 2.5% and 5.0% in the drinking water for 108 weeks. The soap did not induce tumours in the rats (Hiasa et al. 1985).