Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products
4. Nonionic surfactants
Nonionic surfactants are surface-active compounds with hydrophobic and hydrophilic moieties. These surfactants do not ionize in aqueous solutions. Commercial nonionic surfactants are normally a mixture of homologuos structures composed of alkyl chains that differ in the number of carbons and with hydrophilic moieties that differ in the number of ethylene oxide (ethoxylate, EO), propylene oxide (propoxylate, PO) and butylene oxide (butoxylate, BO) units. Nonionic surfactants are widely used in consumer products like, e.g., laundry detergents, cleaning and dishwashing agents, and personal care products. Nonionic surfactants are also widely used in cleaning agents formulated for the industrial and institutional sector. By volume, the most important nonionic surfactants are included in the very versatile group of alcohol ethoxylates and alcohol alkoxylates.
4.1 Alcohol ethoxylates and alcohol alkoxylates
Alcohol ethoxylates (AE) are nonionic surfactants composed of a hydrophobic alkyl chain (fatty alcohol) which is combined with a number of ethoxylate, or ethylene oxide, units via an ether linkage. Alcohol alkoxylates (AA) normally contain both ethylene oxide (EO) and propylene oxide (PO) in their hydrophilic moiety, whereas butylene oxide (BO) is less frequently used. The abbreviation AA has been used to designate nonionic surfactants with a hydrophilic part containing PO (or BO), frequently in combination with EO. AE are used in many types of consumer and industrial products like, e.g., laundry detergents, all-purpose cleaning agents, dishwashing agents, emulsifiers, and wetting agents. AA are used as weakly foaming and foam-mitigating surfactants in household cleaning agents, dishwashing agents and cleaning agents designed for the food industry (Bertleff et al. 1997). Other applications of AA include textile lubricants, agricultural chemicals, and rinse aid formulations.
The nonionic surfactants described in this section include several chemical structures of which a few representative structures are given below.
Lineary primary AE, C13 EO7:
CH3-(CH2)12-O-(CH2-CH2-O-)7H
Iso-C13 branched primary AE, EO7:
![]()
Linear primary AE, C13 EO10, end-capped with n-butylether
CH3-(CH2)12-O-(CH2-CH2-O-)10-CH2-CH2-CH2-CH2-OH
Linear primary AA, EO5, PO4:
![]()
The hydrophobic fatty alcohol usually contains 12-15 carbon atoms, but chain lengths of C9-11 are also used.
4.1.1 Occurrence in the environment
During the 1980s non-quantitative methods for detection of AE were used together with analyses for nonionic surfactants (bismuth iodide active substances, BiAS) to determine the presence of AE in e.g. effluents from wastewater treatment plants. Today, the efforts are directed towards the development of new methods for specific determination of AE at low concentrations in environmental samples. Effluent concentrations of AE in wastewater treatment plants were in the order of 0.006-0.02 mg/l for AE concentrations of 0.19-0.91 mg/l in the influent. A higher AE concentration of 3.4 mg/l in the influent resulted in an effluent concentration of 0.04 mg/l (Holt et al. 1992). A recent environmental monitoring showed that the effluent concentrations of AE from municipal sewage treatment plants in the Netherlands varied between 0.0022 and 0.013 mg/l with an average value of 0.0062 mg/l (Matthijs et al. 1999).
The presence of AE in the aquatic environment has been reported for a Japanese river. The concentration of AE in the river water was below the detection limit of 0.005 mg/l, whereas the concentration in the sediment ranged from 0 to 1.0 mg/kg. A concentration of 0.004 mg/l for C14-15 AE was observed in Ohio River, USA (Holt et al. 1992).
4.1.2 Environmental fate
Biodegradation pathways
Three different mechanisms have been proposed for the biological degradation of AE under aerobic conditions (Marcomini et al. 2000a, 2000b).
| 1. | The first mechanism is a central scission, or ether cleavage, which leads to the formation of fatty alcohols and polyethylene glycols (PEG). The fatty alcohols are first transformed to fatty acids by w -oxidation of the terminal carbon, whereafter the fatty acids are degraded by b -oxidation. The b -oxidation of the fatty acid releases pairs of C-atoms from the carbon chain which are mineralized to CO2. The PEG are degraded via a non-oxidative shortening which releases one glycol unit at a time from the terminus of the PEG, and/or via an oxidative hydrolysis forming monocarboxylated PEG. |
| 2. | The second mechanism is a microbial attack at the terminal carbon of the alkyl chain, via an w -oxidation, followed by a series of b -oxidations. By this mechanism the AE is first transformed to a carboxylated AE (with the carboxylic group at the alkyl chain) which is further degraded via the formation of monocarboxylated and dicarboxylated PEG. |
| 3. | The third mechanism is an w -oxidation of the terminal carbon of the polyethoxylic chain. This mechanism proceeds via the formation of a carboxylated AE (with the carboxylic group at the polyethoxylic chain), which is further degraded via dicarboxylated AE (with carboxylic groups at both alkyl and polyethoxylic ends) and dicarboxylated PEG. |
Recent studies have elucidated the relations between the biodegradation mechanisms and the structure of the AE (Marcomini et al. 2000a, 2000b). The formation of PEG was observed only for a linear AE and an oxo-AE (composed of linear AE and monobranched AE with short 2-alkyl chains, i.e. 2-methyl-, 2-ethyl-, 2-propyl-, and 2-butyl-), whereas only carboxylated AE (with the carboxylic group at the polyethoxylic chain) were detected during biodegradation of a multibranched AE. The absence of carboxylated AE in the experiments with the linear and the monobranched (2-alkyl branched) oxo-AE indicates that the central scission (mechanism 1) was the primary mechanism for the biodegradation of linear and most monobranched AE in the examined commercial mixtures, whereas the multibranched AE was degraded via w -oxidation of the polyethoxylic chain (mechanism 3) (Marcomini et al. 2000a). Biodegradation of an oxo-2-butyl-substituted AE only resulted in carboxylated AE (mainly metabolites with the carboxylic group at the alkyl chain) suggesting that w -oxidation of the alkyl chain was the primary mechanism (mechanism 2). The results obtained with the 2-butyl-substituted AE show that a shift from the central scission to the w ,b -oxidation is introduced when the length of the 2-alkyl branch exceeds three carbon atoms (Marcomini et al. 2000b).
Far less is known about the biodegradation of AA and of end-capped AE. AA containing butoxylate (BO) or propoxylate (PO) groups in their hydrophilic moiety are degraded via cleavages of the hydrophilic chain, which may be either non-oxidative or oxidative like the degradation of PEG. A secondary carbon atom in the hydrophilic moiety, e.g. in PO groups, inhibits the oxidative route (Balson and Felix 1995). End-capped AE are degraded by a combination of w -oxidation of the hydrophilic chain and central hydrophobe-hydrophile scission. The w -oxidation is inhibited by the presence of PO in the hydrophilic chain, whereas the extent of central scission is determined by the degree of 2-alkyl branching (Balson and Felix 1995). The findings in the above-mentioned studies with 2-butyl-substituted AE (Marcomini et al. 2000b) further illustrate the effect of the length of the 2-alkyl substituent.
The anaerobic biodegradation of linear AE is apparently initiated by a stepwise release of C2 units as acetaldehyde to form the corresponding shortened AE and, eventually, a fatty acid (Wagener and Schink 1988). This pathway was recently confirmed in anaerobic assays with a linear pure C12 AE (with 8 EO) and a linear technical C12 AE (with an average of 9 EO), as the first identifiable metabolites were shortened AE and subsequent metabolites included dodecanoic acid and acetic acid. No PEG was observed during the degradation of linear AE, which indicates that central scission of the AE molecule was not the biodegradation mechanism under the applied anaerobic conditions (Huber et al. 2000).
Effects of structure of AE on biodegradability
The biodegradability of the AE is relatively unaffected by the alkyl carbon chain length and the number of EO units but highly affected by the molecular structure of the hydrophobic chain (Dorn et al. 1993). The linear AE are normally easily degraded under aerobic conditions. Only small differences are seen in the time needed for ultimate degradation of linear AE with different alkyl chain lengths. AE with a typical alkyl chain (e.g., C12 to C15) will normally reach more than 60% degradation in standardized tests for ready biodegradability. The length of the EO chain determines the hydrophobicity of the AE and, hence, influences the biodegradability in terms of the bioavailability. Longer EO chains decrease the bioavailability of the AE due to increased hydrophilicity and molecular size, which limits the transport of the molecule through the cell wall (Balson and Felix 1995). For AE containing more than 20 EO units, a reduced rate of biodegradation has been observed (Scharer et al. 1979; Holt et al. 1992).
The biodegradation of branched AE tends to be slower than biodegradation of linear AE. One important observation that may explain this fact is that the b -oxidation is hindered by the branching of the alkyl chain (Holt et al. 1992; Balson and Felix 1995). Furthermore, branching at the C-atom forming the internal ether linkage may hinder the hydrophobe-hydrophile scission (Balson and Felix 1995). The biodegradability of AE depends on degree and structure of the branching. The general trend is that the biodegradation decreases considerably with an increasing branching of the carbon chain (Kaluza and Taeger 1996). A highly branched C13 AE, prepared from 2-propyl-C10 and 2-pentyl-C8 with 46% branching, was not readily biodegradable in the DOC die-away screening test as only 50% of the initial DOC was removed during 28 days (Kaluza and Taeger 1996). The structure of the backbone in the carbon chain also affects the biodegradability. Swisher (1987) found that one single internal methyl group had no effect on the biodegradation compared to the entirely linear AE, whereas two methyl groups decreased the degradation rate markedly, especially if the methyl groups were located at the same carbon resulting in a quaternary structure. The rate of biodegradation of monobranched AE is strongly influenced by the length of the side chain. Although the 60% pass level was fulfilled in the CO2 evolution test (but not the 10-day window), the degradation of an oxo-2-butyl-substituted AE occurred more slowly than the degradation of an oxo-AE blend containing 2-methyl-, 2-ethyl-, 2-propyl-, and 2-butyl side chains. The degradation of the 2-butyl-substituted AE showed a time profile similar to that of a multibranched AE (Marcomini et al. 2000b). Kaluza and Taeger (1996) compared the biodegradability of branched AE based on different carbon chains (all with 7-8 EO units). They found that an iso-C13 AE based on propylene tetramer (four internal methyl groups) did not pass a test for ready biodegradability, whereas an iso-C13 based on butylene trimer (three internal methyl groups) did. The ultimate degradation of iso-C10 based on propylene trimer (three internal methyl groups) also complied with the criteria for ready biodegradability. Kravetz et al. (1991) studied the degradation of a C11-15 AE based on propylene and containing four internal methyl groups as well as a C10-14 AE containing three internal groups (both with 7 EO units). The structures of the two substances were complex as they both contained a quaternary carbon. None of the branched AE passed the criteria for ready biodegradability and no difference in the degradation rates for the two substances was observed.
Aerobic biodegradability
Linear C12-18 AE, containing 5-14 EO units, are ultimately degraded under aerobic conditions. The degradation rate of AE containing more than 20 EO units is slower, although an extensive primary degradation may take place for AE containing up to 50 EO units (Birch 1984). Only a few studies report the fate of AE in wastewater treatment plants. Average concentrations of 0.33 mg/l (0.19-0.47 mg/l) in the influent and 0.009 mg/l (0.006-0.012 mg/l) in the effluent of C14-15 EO7 indicate a removal of 97-98% of the AE during wastewater treatment (Holt et al. 1992). Data on the ultimate aerobic biodegradability of linear AE are shown in Table 4.1. As described previously, the aerobic biodegradation of branched AE depends on the structure of the hydrophobic carbon chain. In general the biodegradability decreases with increasing branching of the alkyl chain, but also the number of internal methyl groups and the presence of quaternary carbon atoms affect the biodegradability of AE. Normally, AE containing a quaternary carbon atom are not readily biodegradable (Table 4.2).
Table 4.1
Ultimate aerobic biodegradability of linear AE.
Compound |
Test |
Result |
Reference |
C9-11 EO8 |
Closed bottle test, 28 d |
80% ThOD |
Madsen et al. 1994 |
C12-14 EO7-8 |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C12-15 EO7 |
BOD, 30 d |
92% ThOD |
Kravetz et al. 1991 |
|
CO2 evolution test, 28 d |
82% ThCO2 |
Madsen et al. 1996b |
C12-15 EO9 |
CO2 evolution test, 28 d |
64-79% ThCO2 |
Kravetz et al. 1991 |
C12-18 EO10-14 |
Closed bottle test, 28 d |
69-86% ThOD |
Sch÷berl et al. 1988 |
C13 EO7-8 |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C13-15 EO7-8 |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C14-15 EO7 |
BOD, 30 d |
83% ThOD |
Kravetz et al. 1991 |
C15 EO7-8 |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C16-18 EO5 |
Closed bottle test, 28 d |
65-75% ThOD |
Sch÷berl et al. 1988 |
C16-18 EO30 |
Closed bottle test, 28 d |
27% ThOD |
Sch÷berl et al. 1988 |
Table 4.2
Ultimate aerobic biodegradability of branched AE.
Compound |
Comments |
Test |
Result |
Reference |
Iso-C10 EO7-8 |
3 internal CH3-groups, highly branched |
Die away screening test, 28 d |
90% DOC |
Kaluza and Taeger 1996 |
Oxo-C11 EO7-8 |
10% branching |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
C10-14 EO7 |
2.9 internal CH3-groups, quaternary C-atom |
BOD, 30 d |
40% ThOD |
Kravetz et al. 1991 |
Oxo-C12 EO5 |
2-butyl-substituted |
CO2 evolution test, 28 d |
> 60% ThCO2 |
Marcomini et al. 2000b |
C12-15 EO7 C12-15 EO18 C12-15 EO30 |
75% primary alcohol |
CO2 evolution test, 28 d |
> 80% ThCO2 |
Scharer et al. 1979 |
Iso-C13 EO7-8 |
3 internal CH3-groups, highly branched |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
Iso-C13 EO7-8 |
4 internal CH3-groups, highly branched |
Die away screening test, 28 d |
62% DOC |
Kaluza and Taeger 1996 |
C11-15 EO7 |
4 internal CH3-groups, quaternary C-atom |
CO2 evolution test, 28 d |
40-50% ThOD |
Kravetz et al. 1991 |
C13 EO7-8 |
< 1 internal CH3-group, 10% branching |
Die away screening test, 28 d |
95% DOC |
Kaluza and Taeger 1996 |
C13 EO7-8 |
1 internal CH3-group, 25% branching |
Die away screening test, 28 d |
95% DOC |
Kaluza and Taeger 1996 |
C13 EO7-8 |
╗ 1 internal CH3-group, 46% branching |
Die away screening test, 28 d |
50% DOC |
Kaluza and Taeger 1996 |
Oxo-C13-15 EO7-8 |
10% branching |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
Oxo-C13-15 EO3-12 |
|
Modified OECD screening test, 28 d |
75% DOC |
Sch÷berl et al. 1988 |
Oxo-C14-15 EO9-20 |
|
Die away screening test, 28 d |
65-75% DOC |
Sch÷berl et al. 1988 |
C15 EO7-8 |
1 internal CH3-group, 25% branching |
Die away screening test, 28 d |
100% DOC |
Kaluza and Taeger 1996 |
The biodegradability of AA generally decreases with an increasing number of PO units in
the hydrophilic part. The results summarized in Table 4.3 confirm this trend as, e.g., the
C12-18 AA containing 6 PO units did not pass the level required for ready
biodegradability whereas the same alcohol containing 2 PO units attained 83% ThOD in the
closed bottle test (Sch÷berl et al. 1988). The general trend for linear AA is
that, apparently, there is a limit of 6-7 PO units in order to qualify for primary
biodegradation (Balson and Felix 1995). However, increased branching of the carbon chain
determines the construction of the hydrophilic part of the surfactant, as fewer PO units
can be tolerated in branched AA in order to comply with the requirement for primary
biodegradability (Naylor et al. 1988). Naylor et al. (1988) showed that a
primary biodegradation > 80% was achieved for the most linear AA (20% branching)
containing up to 3.5 PO units, an AA with 1 internal methyl group containing up to 2.0 PO
units, and an AA with 2 internal methyl groups containing up to 0.4 PO units.
The removal of alcohol propoxylates in German wastewater treatment plants has been reported to be in the range of 73-81% (Holt et al. 1992). If the AA is terminated with PO units the degradation is highly influenced by the branching because the w -hydrophile oxidation is inhibited by the presence of PO. In this case, the level of branching determines the biodegradation which proceeds by the hydrophobe-hydrophile scission (Balson and Felix 1995). This was confirmed by a study showing that the primary biodegradability of an AA containing 6 EO units and 6.5 PO units was 97% for 20% 2-alkyl branching and 10% for 100% 2-alkyl branching (Balson and Felix 1995). Balson and Felix (1995) showed a primary degradation of 83-97% for a C9-11-AE capped with an alkyl group and 80-99% primary degradation of a C9-11-AE capped with an aryl group. Data describing the ultimate biodegradability of end-capped AE are sparse. A C12-14 EO9 and a C12-18 EO10, both end-capped with n-butylether, were confirmed to be readily biodegradable (Table 4.3).
Table 4.3
Ultimate aerobic biodegradability of end-capped AE and AA.
Compound |
Test |
Result |
Reference |
C12-14 EO9, n-butyl-ether (end-capped) |
Closed bottle test, 28 d |
80% ThOD |
Sch÷berl et al. 1988 |
C12-18 EO10, n-butyl-ether (end-capped) |
Manometric respirometry test, 28 d |
98% ThOD |
This study (Appendix; Table A1, Figure A1) |
C8-10 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
C9-11 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
C10-12 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
Iso-C13 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
C13-15 EO6, PO 3 |
OECD ready test |
> pass level |
Bertleff et al. 1997 |
C12-18 EO2.5, PO 6 |
Modified OECD screening test, 28 d |
43% DOC |
Sch÷berl et al. 1988 |
C12-18 EO2.5, PO 6 |
Closed bottle test, 28 d |
36% ThOD |
Sch÷berl et al. 1988 |
C12-18 EO6, PO 2 |
Modified OECD screening test, 28 d |
69% DOC |
Sch÷berl et al. 1988 |
C12-18 EO6, PO 2 |
Closed bottle test, 28 d |
83% ThOD |
Sch÷berl et al. 1988 |
Anaerobic biodegradability
Most of the relatively few studies of the anaerobic biodegradability of AE have been performed with linear AE. Anaerobic biodegradation tests have been performed with various inocula like, e.g., anaerobically digested sludge (Steber and Wierich 1987; Salanitro and Diaz 1995; Madsen et al. 1995; 1996a) and anoxic sediments (Wagener and Schink 1987; Madsen et al. 1995, 1996a; Federle and Schwab 1992). Anaerobic biodegradability tests with diluted digested sludge have either been performed by use of screening methods (e.g., ECETOC 1988; ISO 1995) or by use of 14C-labelled model compounds (e.g., Steber and Wierich 1987). Since the concentration of surfactant in the screening test may inhibit its degradation by anaerobic bacteria, the results from studies using 14C-labelled compounds are generally considered to be of higher value. The results indicate that linear AE are normally mineralized in anaerobically digested sludge. The mineralization observed in experiments with 14C-labelled surfactants suggests that almost complete degradation of linear AE may be expected in anaerobic digesters and that the lower mineralization observed in the screening test was caused by inhibition (Table 4.4). AE end-capped with butylether were either partially mineralized or not degraded in the ISO 11734 screening test (Table 4.4; Appendix). Linear AE were also degraded in anoxic sediments, where a lower mineralization was observed at 22░ C compared to the mineralization at higher temperatures (Table 4.5).
Table 4.4
Ultimate anaerobic biodegradability of AE in digested sludge.
Compound |
Type of test and duration |
Result |
Reference |
C9-11 EO8 |
Measurement of gas production, 35░ C, 40-50 d |
60-83% ThCH4 |
Salanitro and Diaz 1995 |
C9-11 EO8 |
Measurement of gas production, 35░ C, 56 d |
79% ThGP |
Madsen et al. 1996a |
C12-15 EO7 |
Measurement of gas production, 35░ C, 56 d/84d |
38%; 35% ThGP |
Madsen et al. 1996b This study (Appendix; Table A9, Figure A9) |
C18 EO7 |
Measurement of 14CH4 and 14CO2 evolution, 35░ C, 28 d |
84% ThCH4 + ThCO2 |
Steber and Wierich 1987 |
C8 EO5, n-butylether (end-capped) |
Measurement of gas production, 35░ C, 84 d, ISO 11734 |
Inhibition |
This study (Appendix; Table A10, Figure A10) |
C12-18 EO10, n-butyl-ether (end-capped) |
Measurement of gas production, 35░
C, 84 d, |
54% ThGP |
This study (Appendix; Table A11, Figure A11) |
Table 4.5
Ultimate anaerobic biodegradability of AE in sediments.
Compound |
Type of test and duration |
Result |
Reference |
C9-11 EO8 |
Measurement of gas production in freshwater swamp material, 35░ C, 56 d |
77% ThGP |
Madsen et al. 1996a |
C9-11 EO8 |
Measurement of gas production in marine sediment, 35░ C, 56 d |
66% ThGP |
Madsen et al. 1996a |
C10-12 EO7.5 |
Measurement of CH4-production in polluted creek mud, 28░ C, 37 d |
70% ThCH4 |
Wagener and Schink 1987 |
C12 EO8-9 |
Measurement of 14CH4 and 14CO2 evolution in wastewater pond sediment, 22░ C, 87 d |
24-40% ThCH4 + ThCO2 |
Federle and Schwab 1992 |
C12 EO8-9 |
Measurement of 14CH4 and 14CO2 evolution in pond sediment, 22░ C, 87 d |
13% ThCH4 + ThCO2 |
Federle and Schwab 1992 |
C12 EO23 |
Measurements of CH4-production in polluted creek mud, 28░ C, 37 d |
80% ThCH4 |
Wagener and Schink 1987 |
Bioaccumulation
Bioaccumulation of AE in aquatic organisms has been determined only for fish. The majority of the very few data is based on studies with 14C-labelled compounds that do not allow the distinction between the parent compound and metabolites. Because AE are metabolized in aquatic organisms, the bioconcentration factor for the parent compound may well be overestimated in experiments in which 14C-labelled model surfactants are used. By use of 14C-labelled surfactants, whole body concentration ratios have been estimated for four different AE in fish (Table 4.6).
Table 4.6
Whole body BCF values of AE in fish.
Compound/species |
Uptake/ depuration |
BCF |
Reference |
C14 EO7 Bluegill sunfish (Lepomis macrochirus) |
28 d/? |
799* |
Bishop and Maki 1980 |
C12 EO4 Carp (Cyrinus carpio) |
72 h/168 h |
309* |
Wakabayashi et al. 1987 |
C12 EO8 Carp |
72 h/168 h |
222* |
Wakabayashi et al. 1987 |
C12 EO16 Carp |
72 h/168 h |
4.3* |
Wakabayashi et al. 1987 |
C12 EO8 Fathead minnow (Pimephales promelas) |
54-72 h/- |
12.7 |
Tolls 1998 |
C13 EO4 Fathead minnow |
54-72 h/- |
232.5 |
Tolls 1998 |
C13 EO8 Fathead minnow |
54-72 h/- |
29.5-55.0 |
Tolls 1998 |
C14 EO4 Fathead minnow |
54-72 h/24 h |
237.0 |
Tolls 1998 |
C14 EO8 Fathead minnow |
54-72 h/24 h |
56.7-135.2 |
Tolls 1998 |
C14 EO11 Fathead minnow |
54-72 h/24 h |
15.8 |
Tolls 1998 |
C14 EO14 Fathead minnow |
54-72 h/24 h |
< 5 |
Tolls 1998 |
C16 EO8 Fathead minnow |
54-72 h/24 h |
387.5 |
Tolls 1998 |
* BCF values based on radioactivity measurements.
Tolls (1998) combined 14C-techniques and chemical analysis and showed that the parent AE (C13 EO8) was rapidly eliminated by transformation into metabolites, which were eliminated at a slower rate. The bioconcentration factors for C12 EO8 and C13 EO8 were 12.7 and 29.5-55.0, respectively, when the AE were monitored by chemical analysis. The BCF values for C13 EO4 and C14 EO4 were 232.5 and 237.0, respectively. The influence of the hydrophobe chain length was illustrated by BCF values of 56.7 to 135.2 for C14 EO8 and 387.5 for C16 EO8. AE with a relatively high number of EO units, i.e. C14 EO11 and C14 EO14 did not bioaccumulate in fish as indicated by the BCF values of < 5 and 15.8 (Tolls 1998; Table 4.6). The data in Table 4.6 indicate that the more hydrophobic AE (e.g. C13 EO4, C14 EO4, and C16 EO8) have a moderate bioaccumulation potential.
In the study by Tolls (1998) the BCF values ranged from < 5 to 387.5, whereas the uptake rates (k1) varied from 330 to 1660 (l x kg x d-1) and the elimination rates (k2) varied from 3.3 to 59 (d-1). According to the guideline on bioaccumulation studies in fish (OECD 305) the time to 95% steady state conditions can be estimated by the equation t95 = 3.0/k2. Using this equation, the t95 for the AE investigated by Tolls (1998) range from 1.2 to 22 hours. The results obtained by Tolls (1998) indicate that the time to steady state and the BCF for AE increase with decreasing length of the ethoxylate chain (e.g., t95 for C13 EO8 = 2.4 h and BCF = 30-55, and t95 for C13 EO4 = 17.1 h and BCF = 233).
The achievement of steady state conditions for AE (C9-11 EO6, C12-13 EO6.5, and C14-15 EO7) after a relatively short exposure period has also been illustrated by Lizotte et al. (1999) who observed that ‘steady state’ mortality occurred within 240 hours of exposure in the higher exposure concentrations. At the lower exposure concentrations with C9-11 EO6 and C14-15 EO7, the mortality continued, however, throughout the treatment period. For an illustration of the time needed for achievement of maximum toxicity a comparison of toxicity data for AE obtained in short-term and long-term studies is presented in Table 4.7.
Table 4.7
Effects of different exposure periods on the toxicity of AE to fish.
Species |
AE |
LC50 (mg/l) |
Test duration |
Reference |
Fathead minnow (Pimephales promelas) |
C9-11 EO6 |
2.7 |
240 h |
Dorn et al. 1997 |
Fathead minnow, larvae |
C9-11EO6 |
4.87 (4.47-5.26)* |
672 h |
Lizotte et al. 1999 |
Fathead minnow |
C12-13 EO6.5 |
1.3 (0.72-2.7)* |
96 h |
Wong et al. 1997 |
Fathead minnow, larvae |
C12-13 EO6.5 |
2.39 (2.26-2.52)* |
672 h |
Lizotte et al. 1999 |
Fathead minnow |
C14-15 EO7 |
0.63-1.65 |
96 h |
Lewis and Suprenant 1983 |
Fathead minnow, larvae |
C14-15 EO7 |
1.02 (0.94-1.11)* |
672 h |
Lizotte et al. 1999 |
* Parentheses indicate 95% confidence limits.
The data in Table 4.7 indicate that an increase of the exposure period did not lead to lower effect concentrations (LC50) and that maximum toxicity of the AE was achieved after a relatively short exposure period. However, the AE examined by Lizotte et al. (1999) did not include relatively hydrophobic types like, e.g., C13 EO4, C14 EO4, and C16 EO8, for which BCF values above 100 have been determined (Table 4.6).
4.1.3 Effects on the aquatic environment
Many studies have been performed to determine the toxic effects of AE towards aquatic organisms. Extrapolation from laboratory toxicity tests to the environment is obviously not easy for readily biodegradable surfactants, because biodegradation of the compounds in the sewers and in wastewater treatment plants is expected to alter the composition of isomers and homologues. The toxicity of a linear C12-15 EO9 and a branched C11-15 EO7 was investigated after treatment in a continuously activated sludge reactor (Kravetz et al. 1991). Both AE were degraded to products that were not acutely toxic. A higher chronic toxicity was observed for the effluent from the branched AE than from the linear AE. The degradation products were not identified but it was believed that the EO-chain was shortened and, hence, more toxic AE metabolites would have been produced. Garcia et al. (1996) investigated whether the toxicity of AE (C12) was affected by a broad-range or a narrow-range EO distribution. The AE with the narrow-range distribution were less toxic than were the AE with the broad-range EO distribution when the surfactants contained more than 8-10 EO, whereas no differences were observed for a lower degree of ethoxylation. The AE with narrow-range and broad-range EO distribution differed by the presence of a lower amount of free fatty alcohols in the AE with the narrow-range EO distribution. The following paragraphs describe the toxicity of AE and AA towards algae, invertebrates, and fish.
Algae
Algae constitute the group of aquatic organisms which appears to be the most sensitive to AE. The acute toxicity of linear and branched AE to algae is in the same range with EC50 values from 0.05 to 50 mg/l. Besides the differences in chemical structure, the reason for the variation may be due to different test conditions and different test species. For the linear types, the toxicity increases with increasing hydrophobe chain length (comparison of C13 EO7-8 and C15 EO7-8, Table 4.8) and decreasing EO chain length (comparison of C12-14 with 4-13 EO, Table 4.8). The toxicity of AE to algae tends to decrease with increasing degree of branching (Table 4.9). Based on the low EC50 values (ú 1 mg/l), the linear AE of C12-15 EO6-8 are considered as very toxic to algae. When the degree of branching is low (ú 25%), the branched types are also considered very toxic to algae. A C12-14 EO9 end-capped with an n-butyl-group was very toxic to a non-specified alga as the EC50 was 0.3 mg/l (Sch÷berl et al. 1988).
The effect of the carbon chain length and structure on the toxicity to algae was examined for two AA containing 6 EO and 3 PO-groups (Bertleff et al. 1997). It was observed that the toxicity increased with an increasing carbon chain length and that branching of the carbon chain reduced the toxicity (Table 4.10).
Table 4.8
Effects of linear AE to algae.
Species |
AE |
EC50 (mg/l) |
Test Duration |
Reference |
Selenastrum capricornutum |
C12-14 EO4 |
2-4 |
48 h |
Yamane et al. 1984 |
Selenastrum capricornutum |
C12-14 EO9 |
4-8 |
48 h |
Yamane et al. 1984 |
Selenastrum capricornutum |
C12-14 EO13 |
10 |
48 h |
Yamane et al. 1984 |
Nitzschia fonticula |
C12-14 EO9 |
5-10 |
48 h |
Yamane et al. 1984 |
Microcystis aeruginosa |
C12-14 EO9 |
10-50 |
72 h |
Yamane et al. 1984 |
Scenedesmus subspicatus |
C12-14 EO7 |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Selenastrum capricornutum |
C12-15 EO7 |
0.85 (0.84-0.85)* NOEC:0.50 |
72 h |
Madsen et al. 1996b |
Scenedesmus subspicatus |
C13 EO7-8 |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C13-15 EO7-8 |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C14-15 EO6 |
0.09 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
C14-15 EO6 |
0.60 |
96 h |
Lewis and Hamm 1986 |
Navicula pelliculosa |
C14-15 EO6 |
0.28 |
96 h |
Lewis and Hamm 1986 |
Scenedesmus subspicatus |
C15 EO7-8 |
0.05 |
72 h |
Kaluza and Taeger 1996 |
Selenastrum capricornutum |
C12-15 EO9 |
0.7 |
96 h |
Dorn et al. 1993 |
* Parenthesis indicate 95% confidence interval.
Table 4.9
Effects of branched AE to algae.
Species |
AE |
EC50 (mg/l) |
Test Duration |
Reference |
Not indicated |
Oxo-C9-15 EO2-10 |
4-50 |
- |
Sch÷berl et al. 1988 |
Scenedesmus subspicatus |
Iso-C10 EO7-8 (3 internal CH3-groups, highly branched) |
50 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
Iso-C13 EO7-8 (3 internal CH3-groups, highly branched) |
5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
Iso-C13 EO7-8 (4 internal CH3-groups, highly branched) |
5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C13 EO7-8 (< 1 internal CH3-group, 10% branching) |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C13 EO7-8 (1 internal CH3-group, 25% branching) |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C13 EO7-8 (╗ 1 internal CH3-group, 46% branching) |
5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
Oxo-C13-15 EO7-8 (10% branching) |
0.5 |
72 h |
Kaluza and Taeger 1996 |
Scenedesmus subspicatus |
C15 EO7-8 (1 internal CH3-group, 25% branching) |
0.05 |
72 h |
Kaluza and Taeger 1996 |
Selenastrum capricornutum |
C13 EO7 (2 internal CH3-groups, quaternary C-atom) |
7.5 NOEC: 10.0 |
96 h |
Dorn et al. 1993 |
Selenastrum capricornutum |
C11-15 EO7 (4 internal CH3-groups, quaternary C-atom) |
10.0 NOEC: 4.0 |
96 h |
Dorn et al. 1993 |
Table 4.10
Effects of AA and end-capped AE to algae.
Species |
AA |
EC50 (mg/l) |
Test Duration |
Reference |
Algae |
C8-10 EO6, PO3 |
10-100 |
- |
Bertleff et al. 1997 |
Algae |
C9-11 EO6, PO3 |
1-10 |
- |
Bertleff et al. 1997 |
Algae |
C10-13 EO6, PO3 |
1-10 |
- |
Bertleff et al 1997 |
Algae |
Iso-C13 EO6, PO3 |
10-100 |
- |
Bertleff et al. 1997 |
Algae |
C13-15 EO6, PO3 |
0.1-1 |
- |
Bertleff et al. 1997 |
Algae |
C12-14 EO9, butylether (end-capped) |
0.3 |
- |
Sch÷berl et al. 1988 |
Invertebrates
The acute toxicity of AE to invertebrates varies with EC50 values from 0.1 mg/l to more than 100 mg/l for the linear types and from 0.5 mg/l to 50 mg/l for the branched types. The toxicity is species specific and may vary between 0.29 mg/l to 270 mg/l for the same linear AE (Lewis and Suprenant 1983). The most commonly used invertebrates for testing are Daphnia magna and Daphnia pulex, and they are also among the most sensitive invertebrates to AE. Apparently, the toxicity of AE to invertebrates was not related to hydrophobicity as it is the case for algae. Some AE are very toxic to invertebrates, i.e., linear AE of C12-15 EO1-8 and branched AE with a low degree of branching, i.e. < 10-25%. Branching of the alkyl chain reduces the toxicity of AE to invertebrates as also observed for algae. This effect of branching is evident by comparison of the toxicity of the linear C13 AE and C13 AE containing more or less branched alkyl chains (Tables 4.11-4.12). The toxicity of commercial AE was recently determined by using a sperm cell toxicity test with the sea urchin Paracentrotus lividus. The EC50 obtained in this test have proven to be closer to chronic data for all the tested AE. Whereas a fully linear C12 AE exhibited an EC50 of 0.96 mg/l in the sperm cell toxicity test, a fully monobranched C12 AE exhibited an EC50 of 4.0 mg/l. In this case, the alkyl side chain reduced the toxicity of the C12 AE by approximately a factor of 4 (Marcomini et al. 2000c). A C12-14 EO9 end-capped with an n-butyl-group was toxic to daphnids as the acute and chronic EC50 values were 1-2 mg/l and 0.3 mg/l, respectively (Sch÷berl et al. 1988). Sch÷berl et al. (1988) report that an AA with 2-5 EO and 4 PO was toxic to daphnids as the EC50 ranged between 2.4 and 6.0 mg/l.
Table 4.11
Effects of linear AE to invertebrates.
Species |
AE |
EC/LC50 (mg/l) |
Test Duration |
Reference |
Hyalella azteca |
C9-11 EO6 |
14 |
10 d |
Dorn et al. 1997 |
Chironomus tentans |
C9-11 EO6 |
5.7 |
10 d |
Dorn et al. 1997 |
Mysidopsis bahia |
C10 EO4 |
5.6 |
48 h |
Hall et al. 1989 |
Daphnia magna |
C12-13 EO5 C12-13 EO4.5-6 C12-13 EO6.5 |
0.46 (0.39-0.56)* 0.59 (0.42-0.83)* 0.74 (0.63-0.86)* |
48 h |
Wong et al. 1997 |
Daphnia magna |
C12-14 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
Daphnia pulex |
C12-15 EO7 |
0.76 |
48 h |
Salanitro et al. 1988 |
Daphnia magna |
C12-15 EO7 |
1.0-2.0 |
48 h |
Madsen et al. 1996b |
Daphnia magna |
C12-15 EO9 |
1.3 (1.1-1.4)* NOEC: 1.0 |
48 h |
Dorn et al. 1993; Kravetz et al. 1991 |
Daphnia magna |
C13 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
Mysidopsis bahia |
C13 EO10 |
2.2 |
48 h |
Hall et al. 1989 |
Daphnia magna |
C13-15 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
Daphnia magna |
C14 EO1 |
0.83 |
48 h |
Maki and Bishop 1979 |
Daphnia pulex |
C14 EO1 |
0.10 |
48 h |
Maki and Bishop 1979 |
Daphnia magna |
C14-15 EO7 |
0.29-0.4 |
48 h |
Lewis and Perry 1981 |
Paratanytarus parthenogenica (midge) |
C14-15 EO7 |
23 |
48 h |
Lewis and Suprenant 1983 |
Gammarus sp. (amphipod) |
C14-15 EO7 |
3.3 |
48 h |
Lewis and Suprenant 1983 |
Asellus sp. (isopod) |
C14-15 EO7 |
270 |
48 h |
Lewis and Suprenant 1983 |
Dugesia sp. (flatworm) |
C14-15 EO7 |
1.8 |
48 h |
Lewis and Suprenant 1983 |
Dero sp. (oligochaete) |
C14-15 EO7 |
1.7 |
48 h |
Lewis and Suprenant 1983 |
Rhabditis sp. (nematode) |
C14-15 EO7 |
16 |
48 h |
Lewis and Suprenant 1983 |
Daphnia magna |
C14-15 EO13 |
1.2 (0.65-1.9)* |
48 h |
Wong et al. 1997 |
Daphnia magna |
C15 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
Daphnia |
C16-18 EO2-4 |
20-100 |
- |
Sch÷berl et al. 1988 |
Daphnia |
C16-18 EO5-7 |
5-200 |
- |
Sch÷berl et al. 1988 |
Daphnia |
C16-18 EO10-14 |
40-60 |
- |
Sch÷berl et al. 1988 |
Daphnia magna |
C16-18 EO18 |
20 |
48 h |
Talmage 1994 |
Daphnia magna |
C16-18 EO30 |
18 |
48 h |
Talmage 1994 |
* Parentheses indicate 95% confidence intervals.
Table 4.12
Effects of branched AE to Daphnia magna.
AE |
EC50 (mg/l) |
Test Duration |
Reference |
Oxo-C9-15 EO2-10 |
2-10 |
- |
Sch÷berl et al. 1988 |
Oxo-C9-15 EO> 10 |
4-20 |
- |
Sch÷berl et al. 1988 |
Iso-C10 EO7-8 |
50 |
48 h |
Kaluza and Taeger 1996 |
Oxo-C11 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
Iso-C13 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
Iso-C13 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
C13 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
C13 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
C13 EO7-8 |
5 |
48 h |
Kaluza and Taeger 1996 |
Oxo-C13-15 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
C15 EO7-8 |
0.5 |
48 h |
Kaluza and Taeger 1996 |
C13 EO7 |
9.8 |
48 h |
Dorn et al. 1993 |
C11-15 EO7 |
11.6 |
48 h |
Kravetz et al. 1991 Dorn et al. 1993 |
* Parentheses indicate 95% confidence intervals.
Fish
The acute toxicity of AE to fish varies with LC50 values from 0.4 mg/l to more than 100 mg/l for the linear types and from 0.25 mg/l to 40 mg/l for the branched AE (Tables 4.13-4.14). For linear AE the toxicity increases with decreasing EO units (comparison within C12-15 EO7-9 and within C14-15 EO 7-11). C12-15 AE containing 7-11 EO groups are considered to be very toxic to fish (EC/LC50 ú 1 mg/l). There are only few data on the toxicity of branched AE to fish and only oxo-C9-15 EO2-10 is considered very toxic. A C12-14 EO9 end-capped with an n-butyl-group was toxic to fish (species not specified) as the EC50 was 0.5-4.6 mg/l (Sch÷berl et al. 1988). Sch÷berl et al. (1988) report that an AA with 2-5 EO and 4 PO was toxic to fish as the LC50 ranged between 0.7 and 5.7 mg/l.
Table 4.13
Effects of linear AE to fish.
Species |
AE |
LC50 (mg/l) |
Test Duration |
Reference |
Bluegill sunfish |
C10-12 EO6 |
6.4 |
96 h |
Macek and Krzeminski 1975 |
Fathead minnow |
C12-13 EO5 |
1.0 (0.84-1.3)A |
96 h |
Wong et al. 1997 |
Brown trout |
C12-14 EO8 |
0.8 |
96 h |
Reiff et al. 1979 |
Golden orfe |
C12-14 EO8 |
1.8 |
96 h |
Reiff et al. 1979 |
Harlequin fish |
C12-14 EO10-11 |
1.6-2.8 |
96 h |
Reiff et al. 1979 |
Zebra fish |
C12-15 EO7 |
1.0-2.0 |
96 h |
Madsen et al. 1996 |
Bluegill sunfish |
C12-15 EO3 |
1.5 |
96 h |
Macek and Krzeminski 1975 |
Fathead minnow |
C12-15 EO7 |
0.48 |
96 h |
Salanitro et al. 1988 |
Fathead minnow |
C12-15 EO9 |
1.6 (1.3-1.8) A |
96 h |
Dorn et al. 1993; Kravetz et al. 1991 |
Bluegill sunfish |
C12-15 EO9 |
2.1 |
96 h |
Macek and Krzeminski 1975 |
Atlantic salmon |
C12 EO4 |
1.5 25.0 |
96 h |
Wildish 1972 |
Bluegill sunfish |
C13 EO9 |
7.5 |
96 h |
Macek and Krzeminski 1975 |
Rainbow trout |
C14-15 EO7 |
0.78 |
96 h |
Turner et al. 1985 |
Rainbow trout |
C14-15 EO11 |
1.08 |
96 h |
Turner et al. 1985 |
Rainbow trout |
C14-15 EO18 |
5.0-6.3 |
96 h |
Talmage 1994 |
Bluegill sunfish |
C14-15 EO7 |
0.66 |
96 h |
Lewis and Perry 1981 |
Bluegill sunfish |
C14-15 EO7 |
0.7-1.12 |
96 h |
Lewis and Suprenant 1983 |
Fathead minnow |
C14-15 EO7 |
0.63-1.65 |
96 h |
Lewis and Suprenant 1983 |
Fathead minnow |
C14-15 EO13 |
1.0 (0.62-1.9)A |
96 h |
Wong et al. 1997 |
Not indicated |
C16-18 EO2-4 |
> 100 |
- |
Sch÷berl et al. 1988 |
Not indicated |
C16-18 EO5-7 |
3-30 |
- |
Sch÷berl et al. 1988 |
Not indicated |
C16-18 EO10-14 |
1.7-3 |
- |
Sch÷berl et al. 1988 |
Brown trout |
Tallow EO14 |
0.4 |
96 h |
Reiff et al. 1979 |
Golden orfe |
Tallow EO14 |
2.3 |
96 h |
Reiff et al. 1979 |
Harlequin fish |
Tallow EO14 |
0.7 |
96 h |
Reiff et al. 1979 |
A
Parentheses indicate 95% confidence intervals.Table 4.14
Effects of branched AE to fish.
Species |
AE |
LC50 (mg/l) |
Test Duration |
Reference |
Not indicated |
Oxo-C9-15 EO2-10 |
0.25-4 |
- |
Sch÷berl et al. 1988 |
Not indicated |
Oxo-C9-15 EO> 10 |
1-40 |
- |
Sch÷berl et al. 1988 |
Bluegill sunfish |
C11-15 EO9 |
4.6 |
96 h |
Macek and Krzeminski 1975 |
Fathead minnow (Pimephales promelas) |
C13 EO7 (2 internal CH3-groups, quaternary C-atom) |
4.5 |
96 h |
Dorn et al. 1993 |
Fathead minnow |
C11-15 EO7 |
6.1 (5.8-6.3)A |
96 h |
Kravetz et al. 1991 |
A
Parentheses indicate 95% confidence intervals.4.1.4 Effects on human health
Toxicokinetics and acute toxicity
In general, AE are readily absorbed through the skin of guinea pigs and rats and through the gastrointestinal mucosa of rats. AE are quickly eliminated from the body through the urine, faeces, and expired air (CO2) (CIRP 1983; SFT 1991).
Orally dosed AE was absorbed rapidly and extensively in rats, and more than 75% of the dose was absorbed. When applied to the skin of humans, the doses were absorbed slowly and incompletely (50% absorbed in 72 hours). Half of the absorbed surfactant was excreted promptly in the urine and smaller amounts of AE appeared in the faeces and expired air (CO2) (Drotman 1980). The metabolism of C12 AE yields PEG, carboxylic acids, and CO2 as metabolites (SFT 1991). Data describing the acute toxicity of various AE, as indicated by LD50, are presented in Table 4.15. The LD50values after oral administration to rats range from about 1-15 g/kg body weight indicating a low to moderate acute toxicity.
Table 4.15
Acute toxicity (LD50) of AE.
Type of surfactant |
Species |
Route of administration |
LD50 (g/kg body weight) |
Reference |
AE |
Rat |
Oral |
1.6 - > 25 |
Kirk-Otmer 1994 |
AE |
Rat |
Oral |
0.87 - > 25 |
SFT 1991 |
C9-11 EO6 |
Rat |
Oral |
1.4 |
Gingell and Lu 1991 |
C9-11 EO6 |
Rabbit |
Dermal |
< 2 |
Gingell and Lu 1991 |
C12 EO23 |
Rat |
Oral |
8.6 |
CIRP 1983 |
C12 EO23 |
Mouse |
Oral |
3.5 |
CIRP 1983 |
C12 EO4 |
Rat, mouse |
Oral |
5-10 |
CIRP 1983 |
C13 EO6 |
Rat |
Oral |
2.1 |
Benke and Brown 1977 |
C13 EO6 |
Rat |
Dermal |
< 2.0 ml |
Benke and Brown 1977 |
C14 EO7 |
Rat |
Oral |
3.3 |
Benke and Brown 1977 |
C18 EO10 |
Rat |
Oral |
2.91 |
CIRP 1988 |
C18 EO20 |
Rat |
Oral |
1.92 |
CIRP 1988 |
C18 EO2 |
Rat |
Oral |
> 25.1 |
CIRP 1988 |
Oxo-AE |
Rat |
Oral |
< 10 |
HŘls 1993 |
Skin and eye irritation
The ability of nonionic surfactants to cause a swelling of the stratum corneum of guinea pig skin has been studied. C12 AE containing 23 EO groups caused little or no swelling. It was concluded that swelling is due to a reversible conformation change, resulting from coorporative binding of the surfactant (Putterman et al. 1977). The swelling mechanism of the skin involves a combination of ionic binding of the hydrophilic group as well as hydrofobic interactions of the alkyl chain with the substrate. One of the mechanisms of skin irritation caused by surfactants is considered to be denaturation of the proteins of skin. It has also been established that there is a connection between the potential of surfactants to denaturate protein in vitro and their effect on the skin. Nonionic surfactants do not carry any net charge and, therefore, they can only form hydrophobic bonds with proteins. For this reason, proteins are not deactivated by nonionic surfactants, and proteins with poor solubility are not solubilized by nonionic surfactants.
Undiluted C9-11 EO6 was found severely irritant to the rabbit skin. The exposure site was evaluated for erythema and edema using the Draize method of scoring. The Primary Irritation Index (PII) was determined to be 5.3 of a possible 8.0. Less than 2 is mildly irritating, 2 – 5 is moderately irritating and > 5 is severely irritating. According to this system, the undiluted C9-11 EO6 is classified as moderately irritating to rabbit skin (Gingell and Lu 1991). Undiluted C12 EO23 caused no primary irritation when applied to the rabbit skin. No primary cutaneous irritation was observed in clinical studies using 60% C12 EO23 or 100% C12 EO4 (CIRP 1983). C18 AE with either 2, 10 or 20 EO were not irritants when applied to the skin of 200 humans at a concentration of 60% in water (CIRP 1988).
A 1% solution of C13 EO6 and a 10% solution of C14 EO7 were tested for skin irritation using a rabbit closed-patch test. The C13 AE was mildly irritating under these conditions as indicated by a PII score of 1.6, whereas the C14 AE was only moderately irritating with a PII score of 4.2 (Benke and Brown 1977). Undiluted C12 EO23 only caused a slight conjunctival reaction in a Draize eye test with rabbits and no corneal and iridial effects were recorded, both in washed and unwashed eyes, for up to 72 hours (CIRP 1983).
The Draize system for evaluation of eye irritation consists of 8 descriptive ratings with increasing intensity of irritation. The maximum values for scoring are 80 for the cornea, 20 for the conjunctiva and 10 for the iris. The higher the score the more severe the damage. The maximum total score is 110. In Draize eye irritation studies with rabbits, undiluted C12 EO4 was moderately and minimally irritating in the unrinsed and rinsed eye, respectively. Ten and twenty percent solutions were both classified as either slightly or non-irritating to unrinsed and rinsed eyes (CIRP 1983). Undiluted C13 EO6 and C14 EO7 produced severe eye irritation in rabbits. The maximum average scores calculated according to Draize were 59.1 for unrinsed eyes. When a 10% solution was used, or when the eyes were rinsed after application of undeluted AE, a moderate irritation was produced as indicated by a maximum average Draize score of 10 to 35 (Benke and Brown 1977).
Aqueous concentrations of up to 60% of C18 AE with either 2EO or 10EO were mildly and minimally irritating to the rabbit eye, respectively. In rabbits C18 EO10 was practically non-irritating to the eye, whereas C18 EO2 and C18 EO20 were minimally irritating to the eye with no water rinse. All of the three C18 AE were non-irritating to the eyes when the eyes were rinsed with water. No irritation of the cornea and iris was observed in rinsed eyes (CIRP 1988).
Sensitization
A 1% w/v aqueous dilution of a C9-11 EO6 was not a skin sensitizer in a guinea pig skin sensitization assay according to the Buehler method. It is an EEC accepted allergy test method and is mentioned in the OECD test guideline No. 406, "Skin Sensitization" (Gingell and Lu 1991). No evidence of sensitization was reported when a 25% solution of C12 EO23 was used in a repeated insult patch test on 168 subjects. The surfactant was applied at 48 hours intervals three times per week for 3 weeks. Then a 3 week non-treatment period followed before the subjects were challenged using the same procedure. A C12 EO4 did not produce sensitization when applied at 100% to 50 subjects in an other patch test. No reactions were observed after the induction or the challenge application (CIRP 1983).
Subchronic and chronic toxicity
A diet containing 1% C14 EO7 or C13 EO6 produced increased liver–to-body weight ratios after administration to rats for 91 days, although, histologically, these livers appeared normal (Brown and Benke 1977). Systemic toxicity of C12 EO4 was not observed in subchronic (21 days) and chronic (3 months, twice daily) dermal tests with diluted formulations (6% in 52% aqueous ethanol solution) on rabbits (CIRP 1983). No observable systemic toxicity was produced in 4 or 13 week subchronic percutaneous toxicity studies after repeated dermal doses (up to 50 mg/day) of C13 EO6 and C14 EO7 in rabbits (Brown and Benke 1977; Talmage 1994).
Reproductive toxicity
The possible adverse effects of dermally applied C9-11 EO6 on the reproductive performance of rats and their offspring over two generations were evaluated by monitoring fertility, gestation, lactation, pup growth and survival. The rats were exposed unoccluded, three days per week, to 0.1 ml/kg body weight of concentrations of 1, 10 and 25% AE. No effects on the reproductive performance or on the growth and development of the offspring were detected (Gingell and Lu 1991). No teratogenic or embryotoxic effects were seen when rats were treated topically with 6% C12 EO4 in 52% ethanol on day 6 to day 15 of gestation (CIRP 1983).
Mutagenicity
There was no evidence of mutagenicity of C9-11 EO6 when tested in the Ames test (gene mutation test). The mutagenic response was investigated in Salmonella typhimurium strains by evaluation of their ability to induce base-pair substitution and frame-shift mutations (Gingell and Lu 1991). Data on genotoxicity were collected in a survey of nine short-term genotoxicity testing for many different types of nonionic surfactants. None of these data indicated any mutagenic potential of AE (Yam et al. 1984; Dean 1985; Zeiger and Anderson 1988).
Classification
Alcohol ethoxylates are according to CESIO (2000) classified as Irritant or Harmful depending on the number of EO-units:
| EO < 5 gives Irritant (Xi) with R38 (Irritating to skin) and R41 (Risk of serious damage to eyes) | |
| EO > 5-15 gives Harmful (Xn) with R22 (Harmful if swallowed) – R38/41 | |
| EO > 15-20 gives Harmful (Xn) with R22-41 | |
| > 20 EO is not classified (CESIO 2000) | |
| Oxo-AE, C13 EO10 and C13 EO15, are Irritating (Xi) with R36/38 (Irritating to eyes and skin) (HŘls 1993). |
AE are not included in Annex 1 of the list of dangerous substances of the Council Directive 67/548/EEC.
4.2 Block copolymers
Block copolymers are weakly foaming substances that have found applications within areas as detergents (foam-mitigating agents), wetting agents, emulsifiers, textile lubricants, and agricultural chemicals. Block copolymers are now being replaced in many household detergents by alcohol alkoxylates (AA) that comply better with the current requirements for biodegradability. The block copolymers consist of long chains of ethylene oxide (EO) and propylene oxide (PO) units. Contrary to other nonionic surfactants, the block copolymers do not contain a hydrophobic moiety based on a fatty alcohol. Instead, the PO units function as the hydrophobic part which establish surface active properties in combination with the more hydrophilic EO units.
4.2.1 Environmental fate
Aerobic biodegradability
The block copolymers fail to meet the requirements for ready biodegradability and also their primary biodegradability may be limited. The biodegradation mechanisms are supposed to be similar to the mechanisms responsible for the degradation of the hydrophilic part of AA: The EO/PO chain is degraded from the terminus by sequential cleavage of individual glycol units. Inclusion of PO units may reduce the biodegradability due to the possible presence of a secondary C-atom which is known to inhibit the degradation (Balson and Felix 1995). The high molecular weight of the copolymers increases the time needed for biodegradation, as the degradation proceeds by terminal attack only. Furthermore, the molecular weight also limits the transport through the bacterial cell wall and thus limits the intracellular degradation. Primary biodegradability of copolymers varies between 5 and 58%, the higher values representing polymers with a high content of EO (Balson and Felix 1995). Removal of EO/PO block polymers was found to be 7% in a confirmatory test and 2-4% in a coupled unit’s test (Holt et al. 1992).
4.2.2 Effects on the aquatic environment
Block copolymers are some of the least toxic types of nonionic surfactants. Aquatic toxicity of block copolymers is reported with EC/LC50 values of more than 100 mg/l for fish and invertebrates (Sch÷berl et al. 1988). In spite of the limited biodegradability, the block copolymers are generally not considered to cause adverse effects in aquatic environments at concentrations below 100 mg/l.
4.3 Alkyl glycosides and glucose amides
Alkyl polyglycosides (APG) and fatty acid glucose amides (FAGA) are used in household products like cleaning agents, liquid dishwashing agents and laundry detergents. APG are composed of a linear fatty alcohol which is bound to the C-1 carbon of the glucose molecule by a glycosidic bond. Commercial APG mixtures usually have an average degree of polymerization (DP) of approximately 1.4 moles of glucose per mole of fatty alcohol.
APG have the following structure:
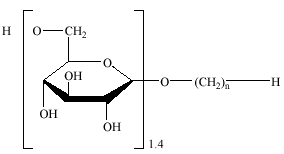
The alkyl chain usually contains either 8-10 or 12-14 carbons (Steber et al. 1995).
FAGA have the following structure:
R-CH2-CO-N-(CH3)-CH2-(CHOH)4-CH2-OH
No data were found on the occurrence of APG or FAGA in the environment.
4.3.1 Environmental fate
Effects of structure of APG on biodegradability
The effects of the APG structure on the aerobic degradation pathway have not been described and no metabolites have been identified. Under strictly anoxic conditions, a branched C8 APG was only partially degraded in contrast to the extensive anaerobic degradation of linear APG (Madsen et al. 1996b). Similarly, the pathways by which FAGA biodegrades are not yet known.
Aerobic biodegradability
According to the results obtained in OECD tests for ready biodegradability, APG with alkyl chain lengths from C8 to C16 are readily biodegradable (Table 4.16). With the exception of the C8 APG, all APG in Table 4.16 are based on linear alkyl chains. Ultimate aerobic biodegradability of C12-14 APG was also tested in an OECD confirmatory test showing 96-100% removal of DOC (Sch÷berl 1997). A similar high biodegradability of C12-14 APG was seen in a coupled units test in which a 89% DOC removal was achieved (Steber et al. 1995). The primary biodegradation of APG was also rapid in the OECD confirmatory test as indicated by a specific analysis of APG (Steber et al. 1995). Ultimate biodegradation without formation of stable metabolites was confirmed in a modified coupled units test. In this test, the effluent from the treatment unit was circulated to detect any possible accumulation of non-readily degradable substances. The C12-14 APG reached 100% of DOC removal indicating that the APG was completely mineralized without any accumulation of metabolites (Steber et al. 1995).
The ready biodegradability of a special type of glycoside surfactant, an ethyl glycoside fatty acid 6-O monoester (C12) (EGE), was examined in the CO2 evolution test and the closed bottle test (Table 4.16). The C12 EGE was degraded more rapidly than the other examined surfactants (C12-14 APG, C8 branched APG, and C12-15 AE), and, for C12 EGE, 65% of ThOD was reached after only 5 days in the closed bottle test (Madsen 1996b). The C12 EGE has previously been succesfully applied in pilot-scale laundry detergents (Andresen et al. 1995), but, to our knowledge, no commercial household products containing this type of surfactant are available. A C12-14 glucose amide (C12-14 FAGA) reached 89 and 86% of ThCO2, respectively, for substrate concentrations of 10 and 20 mg/l (Stalmans et al. 1993; Table 4.16). In an activated sludge mineralization experiment with a 14C-labelled C12 FAGA, 89% of the added 14C was recovered as 14CO2 after 28 days and the mineralization half-life was calculated to 1.26 days (Stalmans et al. 1993).
Table 4.16
Ultimate aerobic biodegradability of alkyl glycosides and glucose amides.
Compound |
Test |
Result |
Reference |
C8 branched APG |
CO2 evolution test, 28 d |
78% ThCO2 |
Madsen et al. 1996b |
C8 branched APG |
Closed bottle test, 28 d |
68% ThOD |
Madsen et al. 1996b |
C8-10 APG |
Modified OECD screening test, 28 d |
94% DOC |
Steber et al. 1995 |
C8-10 APG |
Closed bottle test, 28 d |
81-82% ThOD |
Steber et al. 1995 |
C8-16 APG |
Modified OECD screening test, 28 d |
100% DOC |
Garcia et al. 1997 |
C8-16 APG |
Closed bottle test, 30 d |
80% ThOD |
Garcia et al. 1997 |
C9-11 APG |
Modified OECD screening test, 28 d |
100% DOC |
Garcia et al. 1997 |
C9-11 APG |
Closed bottle test, 30 d |
94% ThOD |
Garcia et al. 1997 |
C12-14 APG |
Die away screening test, 28 d |
95-96% DOC |
Steber et al. 1995 |
C12-14 APG |
Modified OECD screening test, 28 d |
90-93% DOC |
Steber et al. 1995 |
C12-14 APG |
Closed bottle test, 28 d |
73-88% ThOD |
Steber et al. 1995 |
C12-14 APG |
Closed bottle test, 28 d |
67% ThOD |
Madsen et al. 1996b |
C12-14 APG |
CO2 evolution test, 28 d |
81% ThCO2 |
Madsen et al. 1996b |
C12-16 APG |
Modified OECD screening test, 28 d |
100% DOC |
Garcia et al. 1997 |
C12-16 APG |
Closed bottle test, 30 d |
78% ThOD |
Garcia et al. 1997 |
C12 EGE |
CO2 evolution test, 28 d |
78% ThCO2 |
Madsen et al. 1996b |
C12 EGE |
Closed bottle test, 28 d |
80% ThOD |
Madsen et al. 1996b |
C12-14 FAGA |
CO2 evolution test, 35 d |
86; 89% ThCO2 |
Stalmans et al. 1993 |
Anaerobic biodegradability
Several studies have shown that APG with a linear alkyl chain are ultimately biodegradable in the absence of molecular oxygen (Table 4.17). The anaerobic biodegradation of these surfactants is normally rapid and may exceed 60% of ThGP within 28 days (Madsen et al. 1995). Also the glycoside monoesters, C10 and C12 EGE, were extensively biodegraded in an anaerobic screening test with digested sludge (Table 4.17). The biodegradability of alkyl glycosides has also been determined in screening tests with anoxic sediments. By using material from a freshwater swamp as the inoculum, the ultimate biodegradability during 56 days at 35░ C reached 76% of ThGP for C12-14 APG, 83% of ThGP for C10 EGE, and 89% of ThGP for C12 EGE. In a similar test with an inoculum obtained from a marine sediment, the biodegradability during 56 days at 35░ C attained 79% of ThGP for C10 EGE (Madsen 1996a). Branching of the alkyl chain may limit the anaerobic mineralization as indicated by the low biodegradability of a branched C8 APG (Table 4.17).
Table 4.17
Ultimate anaerobic biodegradability of alkyl glycosides in digested sludge.
Compound |
Test |
Result |
Source |
C8 branched APG |
Measurement of gas production, 35░ C, 56 d |
22% ThGP |
Madsen et al. 1996b |
C8-10 APG |
Measurement of gas production, 35░ C, 56 d ECETOC test |
95% ThGP |
Steber et al. 1995 |
C12-14 APG |
Measurement of gas production, 35░ C, 56 d ECETOC test |
84% ThGP |
Steber et al. 1995 |
C12-14 APG |
Measurement of gas production, 35░ C, 56 d |
72; 92% ThGP |
Madsen et al. 1996b; Madsen et al. 1996a |
C10 EGE |
Measurement of gas production, 35░ C, 56 d |
96% ThGP |
Madsen et al. 1996a |
C12 EGE |
Measurement of gas production, 35░ C, 56 d |
82% ThGP |
Madsen et al. 1996a |
Bioaccumulation
No experimental data describing the bioaccumulation potential of APG or FAGA were found in the literature.
4.3.2 Effects on the aquatic environment
The aquatic toxicity of alkyl glycosides and glucose amides is characterized by EC/LC50 values in the range from 2.5 to more than 100 mg/l with the lowest toxicity for the short-chained APG. With EC/LC50 values of 2.5-12 mg/l, C12-14 APG are considered toxic to aquatic organisms, whereas C8-10 APG have a lower toxicity with EC/LC50 │ 20 mg/l. The EC/LC50 values for algae, crustaceans and fish were between 11 and 38 mg/l for C12 EGE and between 2.9 and 57 mg/l for FAGA with C12 to C14 alkyl chains (Table 4.18-4.20).
Table 4.18
Effects of alkyl glycosides and glucose amides to algae.
Compound |
Species |
EC50 (mg/l) |
Duration |
Reference |
C8 branched APG |
Selenastrum capricornutum |
1,543 (1,474-1,621)* |
72 h |
Madsen et al. 1996b |
C8 branched APG |
Selenastrum capricornutum |
NOEC: 100 |
72 h |
Madsen et al. 1996b |
C8-10 APG |
Scenedesmus subspicatus |
21 |
72 h |
Steber et al. 1995 |
C8-10 APG |
Scenedesmus subspicatus |
NOEC: 5.7 |
72 h |
Steber et al. 1995 |
C8-16 APG |
Scenedesmus subspicatus |
14.8 |
96 h |
Henkel KgaA |
C8-16 APG |
Scenedesmus subspicatus |
NOEC: 5.0 |
96 h |
Henkel KgaA |
C12-14 APG |
Scenedesmus subspicatus |
6.0 |
72 h |
Steber et al. 1995 |
C12-14 APG |
Scenedesmus subspicatus |
NOEC: 2.0 |
72 h |
Steber et al. 1995 |
C12-14 APG |
Selenastrum capricornutum |
11 (10-13)* |
72 h |
Madsen et al. 1996b |
C12-14 APG |
Selenastrum capricornutum |
NOEC: 3.1 |
72 h |
Madsen et al. 1996b |
C12 EGE |
Selenastrum capricornutum |
38 (37-38)* |
72 h |
Madsen et al. 1996b |
C12 EGE |
Selenastrum capricornutum |
NOEC: 11 |
72 h |
Madsen et al. 1996b |
C12 FAGA |
Selenastrum capricornutum |
57 (50-64)* |
96 h |
Stalmans et al. 1993 |
C12 FAGA |
Selenastrum capricornutum |
NOEC: 21 |
96 h |
Stalmans et al. 1993 |
C12-14 FAGA |
Selenastrum capricornutum |
13 (12-14)* |
96 h |
Stalmans et al. 1993 |
C12-14 FAGA |
Selenastrum capricornutum |
NOEC: 5.6 |
96 h |
Stalmans et al. 1993 |
C14 FAGA |
Selenastrum capricornutum |
3.9 (2.5-6.4)* |
96 h |
Stalmans et al. 1993 |
C14 FAGA |
Selenastrum capricornutum |
NOEC: 2.9 |
96 h |
Stalmans et al. 1993 |
* Parentheses indicate 95% confidence intervals.
Table 4.19
Effects of APG to Daphnia magna.
Compound |
EC50 (mg/l) |
Duration |
Reference |
C8 branched APG |
557 (465-717)* |
48 h |
Madsen et al. 1996b |
C8-10 APG |
20 |
48 h |
Steber et al. 1995 |
C8-16 APG |
85 |
48 h |
Henkel KgaA |
C12-14 APG |
12 (10-14)* |
48 h |
Madsen et al. 1996b |
C12-14 APG |
7.0 |
48 h |
Steber et al. 1995 |
C12-14 APG |
NOEC: 1.0 |
21 d (reprod.) |
Steber et al. 1995 |
C12 EGE |
23 (21-25)* |
48 h |
Madsen et al. 1996b |
C12 FAGA |
44 (38-53)* |
48 h |
Stalmans et al. 1993 |
C12-14 FAGA |
18 (16-21)* |
48 h |
Stalmans et al. 1993 |
C12-14 FAGA |
NOEC: 4.3 (survival) |
21 d |
Stalmans et al. 1993 |
C14 FAGA |
5.0 (3.3-9.2)* |
48 h |
Stalmans et al. 1993 |
* Parentheses indicate 95% confidence intervals.
Table 4.20
Effects of APG to fish.
Compound |
Species |
LC50 (mg/l) |
Duration |
Reference |
C8 branched APG |
Zebra fish (Brachydanio rerio) |
558 |
96 h |
Madsen et al. 1996b |
C8-10 APG |
Zebra fish |
101 |
96 h |
Steber et al. 1995 |
C8-16 APG |
Zebra fish |
7.8 |
96 h |
Henkel KgaA |
C12-14 APG |
Zebra fish |
2.5-5.0 |
96 h |
Madsen et al. 1996b |
C12-14 APG |
Zebra fish |
3.0 |
96 h |
Steber et al. 1995 |
C12-14 APG |
Zebra fish |
NOEC: 1.8 |
28 d |
Steber et al. 1995 |
C12 EGE |
Zebra fish |
11-17 |
96 h |
Madsen et al. 1996b |
C12 FAGA |
Fathead minnow (Pimephales promelas) |
39 (31-51) |
96 h |
Stalmans et al. 1993 |
C12-14 FAGA |
Zebra fish |
7.5 |
96 h |
Stalmans et al. 1993 |
C14 FAGA |
Fathead minnow |
2.9 (2.4-3.7) |
96 h |
Stalmans et al. 1993 |
4.3.3 Effects on human health
Acute toxicity
The toxicity of APG by oral and dermal administration is low (Table 4.21).
Table 4.21
Acute toxicity (LD50) of APG.
Compound |
Species |
Application |
LD50 (g/kg body weight) |
Reference |
C10 APG |
Rat |
Oral |
> 10 |
Hughes and Lew 1970 |
C8 alkyl glycoside |
Rat |
Oral |
> 2 |
Akzo Nobel 1998 |
C8 alkyl glycoside |
Rabbit |
Dermal |
> 2 |
Akzo Nobel 1998 |
n-Octadecyl-9.0-glycoside |
Rat |
Oral |
> 35.5 |
Hughes and Lew 1970 |
Skin and eye irritation
Patch test carried out on 10 volunteers at concentrations up to 10% active matter of a C10 APG showed no skin irritation (Hughes and Lew 1970).
Classification
Alkyl glycosides are considered non-irritating to skin, but irritating to eyes at very high concentrations. A general classification of a 65% C8 alkyl glycoside solution according to the Substance Directive 67/548/EEC is Irritating (Xi) with the risk phrase R41 (Risk of serious damage to the eyes) or R36 (Irritating to the eyes) (Akzo Nobel 1998).
Alkyl glycosides are not included in Annex 1 of the list of dangerous substances of Council Directive 67/548/EEC.
4.4 Fatty acid amides
Fatty acid amides (FAA) are used in hair shampoo, liquid soaps, shaving creams and other personal care products. FAA consist of a fatty acid, usually derived from coconut oil, which is linked to an amide group by a C-N bond. The amide may either be monoethanolamide (MEA), diethanolamide (DEA), or monoisopropanolamide (MIPA). Representative structures of FAA are indicated below.
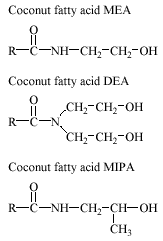
The alkyl chain usually contains 12 to 18 carbon atoms.
4.4.1 Environmental fate
Aerobic biodegradability
Most fatty acid amides (FAA), like e.g. the widely used cocodiethanolamide (cocoamide DEA) and cocomonoethanolamide (cocoamide MEA), are ultimately degraded in the OECD tests for ready biodegradability. The available data describing the biodegradability of the ethoxylated FAA are contradictory. Data cited by Sch÷berl et al. (1988) indicate that these surfactants do not pass the criteria for ready biodegradability, whereas the opposite is the case for data obtained from Akzo Nobel (1999a, 1999b) (Table 4.22).
Table 4.22
Ultimate aerobic biodegradability of FAA.
FAA |
Test |
Result |
Reference |
Cocoamide MEA |
Closed bottle test, 30 d |
82% ThOD |
IUCLID 2000 |
Cocoamide DEA |
Closed bottle test, 30 d |
71% ThOD |
IUCLID 2000 |
C12-18 amide DEA |
Modified OECD screening test, 28 d |
74% DOC |
Sch÷berl et al. 1988 |
C18 amide DEA |
Coupled units test |
87% DOC |
Sch÷berl et al. 1988 |
C12-14 amide MEA EO 4 |
Closed bottle test, 28 d |
47% ThOD |
Sch÷berl et al. 1988 |
C12-14 amide MEA EO 10 |
Closed bottle test, 28 d |
35% ThOD |
Sch÷berl et al. 1988 |
C12-14 amide MEA E05 |
CO2 evolution test, 28 d |
> 60% ThCO2 |
Akzo Nobel 1999a |
C12-14 amide MEA E012 |
CO2 evolution test, 28 d |
> 60% ThCO2 |
Akzo Nobel 1999b |
The primary biodegradability of FAA during 19 days attained 91-100% for C12
amide MEA, 90-99% for C12 amide DEA, and 90-98% for the ethoxylated C12
amide DEA EO5 (Swisher 1987). Primary biodegradation of C18 amide MEA EO6
attained 97-98% removal in an OECD-confirmatory test (Sch÷berl 1997).
Anaerobic biodegradability
The anaerobic biodegradability of FAA has been examined for cocoamide MEA by using the ECETOC screening test (ECETOC 1988). Ultimate anaerobic biodegradability of cocoamide MEA reached 79% of the theoretical gas production, ThGP, during incubation of diluted digested sludge for 42 days at 35░ C (IUCLID 2000). By use of the ISO 11734 screening test, which corresponds to the ECETOC method, the ultimate anaerobic biodegradability of cocoamide MEA attained 81% during 56 days (Appendix; Table A12, Figure A12).
Bioaccumulation
No experimental data describing the bioaccumulation potential of fatty acid amides were found in the literature.
4.4.2 Effects on the aquatic environment
The aquatic toxicity of FAA has been determined for species representing the three trophic levels algae, invertebrates, and fish. Cocoamide DEA appears to be more toxic to aquatic organism than cocoamide MEA.
An exceptionally high toxicity of cocoamide MEA was reported for two tests with the green alga Scenedesmus subspicatus as the 96 h-EC50 were 1.0 and 1.1 mg/l (IUCLID 2000). More recent tests with a pure cocoamide MEA (purity │ 95.5% C12-18, personal communication with Jørgen Hyldgaard, Plum Hudsikkerhed) gave EC50 values of 16.6 mg/l for Scenedesmus subspicatus and 17.8 mg/l for Pseudokirchneriella subcapitata (formerly Selenastrum capricornutum) (Plum Hudsikkerhed 2000a; 2000b). The latter data indicate that the toxicity of cocoamide MEA to algae are not markedly higher than the toxicity to daphnids and fish, and EC50 values above 10 mg/l are probably more representative for the toxicity towards algae. The ethoxylated FAA show the same level of aquatic toxicity as the non-ethoxylated FAA (Table 4.23-4.24).
Table 4.23
Aquatic toxicity of FAA to algae.
FAA |
Species |
EC/LC50(mg/l) |
Duration |
Reference |
Cocoamide MEA |
Scenedesmus subspicatus |
1.0; 1.1 |
96 h |
IUCLID 2000 |
Cocoamide MEA |
Scenedesmus subspicatus |
Biomass |
72 h |
Plum Hudsikkerhed 2000a |
Cocoamide MEA |
Pseudo- kirchneriella subcapitata |
Biomass |
72 h |
Plum Hudsikkerhed 2000b |
Cocoamide DEA |
Scenedesmus subspicatus |
2.2; 2.3 |
96 h |
IUCLID 2000 |
C12-14 amide MEA EO5 |
Scenedesmus subspicatus |
20 |
96 h |
Akzo Nobel 1999a |
C12-14 amide MEA EO4 |
Scenedesmus subspicatus |
14 |
72 h |
Akzo Nobel 1999c |
A
Parentheses indicate 95% confidence intervals.Table 4.24
Aquatic toxicity of FAA to crustaceans and fish.
FAA |
Species |
EC/LC50 (mg/l) |
Duration |
Reference |
Cocoamide MEA |
Daphnia magna |
24.8; 37.5 NOEC: 10.1; 11 |
24 h |
IUCLID 2000 |
Cocoamide MEA |
Zebra fish (Brachydanio rerio) |
28.5; 31 NOEC: 10.1; 11 |
96 h |
IUCLID 2000 |
Cocoamide DEA |
Daphnia magna |
4.2; 5.4 NOEC: 2.5; 2.8 |
24 h |
IUCLID 2000 |
Cocoamide DEA |
Daphnia magna |
2.4 |
48 h |
IUCLID 2000 |
Cocoamide DEA |
Zebra fish |
3.6; 4.0 NOEC: 2.5; 2.8 |
96 h |
IUCLID 2000 |
Cocoamide DEA |
Rice fish (Oryzias latipes) |
10.8-13.8 |
24 h |
IUCLID 2000 |
C12-14 amide MEA EO4 |
Daphnia sp. |
10-100 |
- |
Sch÷berl et al. 1988 |
C12-14 amide MEA EO4 |
Fish |
4-20 |
- |
Sch÷berl et al. 1988 |
C12-14 amide DEA EO4 |
Daphnia sp. |
2-3 |
- |
Sch÷berl et al. 1988 |
4.4.3 Effects on human health
Acute toxicity
The fatty acid diethanolamides all have a low oral toxicity (Table 4.25).
Table 4.25
Acute toxicity (LD50) of FAA.
FAA |
Species |
Application |
LD50 (g/kg body weight) |
Reference |
Cocoamide DEA |
Rat |
Oral |
12.2 |
CIRP 1996 |
Lauramide DEA |
Rat |
Oral |
2.7 |
CIRP 1986 |
Linoleamide DEA |
Rat |
Oral |
> 5 |
CIRP 1986 |
Oleamide DEA |
Rat |
Oral |
> 10 |
CIRP 1986 |
Skin and eye irritation
A 30% cocoamide DEA solution was a moderate skin irritant in rabbits. Test sites were scored for irritation according to Draize, and the Primary Irritation Index (PII) was 3.1 (maximum irritation is indicated by the score of 8). In products intended for prolonged contact with the skin, the concentration of cocoamide DEA should not exceed 5% (CIRP 1996). Low concentrations (0.6%) of cocoamide DEA are severely irritating to the eyes of rabbits. The substance was tested according to a modified Draize eye irritaton test (CIRP 1996).
Sensitization
Several studies of the sensitization potential of cocoamide DEA indicate that this FAA induces occupational allergic contact dermatitis and a number of reports on skin allergy patch testing of cocoamide DEA have been published. These tests indicate that allergy to cocoamide DEA is becoming more common (Hindson and Lawlor 1983; DeGroot et al. 1987; Wall and Gebauer 1991; Pinola et al. 1993; Fowler 1998).
Carcinogenicity
Alkanolamides are manufactured by condensation of diethanolamine and the methylester of long chain fatty acids. The alkanolamides are susceptible to nitrosamine formation which constitutes a potential health problem. Nitrosamine contamination is possible either from pre-existing contamination of the diethanolamine used to manufacture cocoamide DEA, or from nitrosamine formation by nitrosating agents in formulations containing cocoamide DEA (Pinola et al. 1993). According to the Cosmetic Directive (2000) cocoamide DEA must not be used in products with nitrosating agents because of the risk of formation of N-nitrosamines. The maximum content allowed in cosmetics is 5% fatty acid dialkanolamides, and the maximum content of N-nitrosodialkanolamines is 50 Ág/kg. The preservative 2-bromo-2-nitropropane-1,3-diol is a known nitrosating agent for secondary and tertiary amines or amides. Model assays have indicated that 2-bromo-2-nitropropane-1,3-diol may lead to the N-nitrosation of diethanolamine forming the carcinogenic compound, N-nitrosodiethanolamine which is a potent liver carcinogen in rats (IARC 1978).
Mutagenicity
Several FAA have been tested in short-term genotoxicity assays. No indication of any potential to cause genetic damage was seen (Yam et al. 1984). Lauramide DEA was tested in mutagenicity assays and did not show mutagenic activity in Salmonella typhimurium strains or in hamster embryo cells (Inoue and Sunakawa 1980). Cocoamide DEA was not mutagenic in strains of Salmonella typhimurium when tested with or without metabolic activation (Zeiger and Anderson 1988).
Classification
Cocoamide DEA is a possible occupational allergen. Nitrosamine contamination is possible when fatty acid diethanolamides are used together with nitrosating agents.
Fatty acid diethanolamides (C8-CŻ8) are classified by CESIO as Irritating (Xi) with the risk phrases R38 (Irritating to skin) and R41 (Risk of serious damage to eyes). Fatty acid monoethanolamides are classified as Irritant (Xi) with the risk phrases R41 (CESIO 2000).
Fatty acid amides are not included in Annex 1 of the list of dangerous substances of Council Directive 67/548/EEC.