Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products
5. Cationic surfactants
Cationic surfactants are surface-active compounds with at least one hydrophobic alkyl
chain and a hydrophilic group carrying a positive charge. Cationic surfactants are
positively charged in aqueous solutions. Of the cationic surfactants especially the
quaternary ammonium compounds are used in commercial products. The quaternary ammonium
compounds are characterized by a positively charged quaternary nitrogen atom. Commercial
raw materials are normally derived from natural oils which implies that homologous
mixtures of surfactants with different alkyl chain lengths are used in the products. In
household products, the cationic surfactants are primarily applied in fabric softeners,
hair conditioners, and other hair preparations. Other applications of cationic surfactants
include disinfectants and biocides, emulsifiers, wetting agents, and processing additives.
By volume, the most important cationic surfactants in household products are the alkyl
ester ammonium salts that are used in fabric softeners.
This Chapter focuses entirely on quaternary ammonium compounds. As the surfactants in this group may have long and complicated names, a number of abbreviations are used in the present Chapter.
| ATMAC: | Alkyltrimethylammonium chlorides |
| ATMAB: | Alkyltrimethylammonium bromides |
| DADMAC: | Dialkyldimethylammonium chlorides |
| DADMAMS: | Dialkyldimethylammonium methyl sulfates |
| DSDMAC: | Distearyldimethylammonium chlorides |
| DTDMAC: | Ditallowdimethylammonium chlorides |
| ADMBAB: | Alkyldimethylbenzylammonium bromides |
| ADMBAC: | Alkyldimethylbenzylammonium chlorides |
| EQ: | Esterquats |
| DEQ: | Diesterquats |
| DEEDMAC: | Diethyl ester dimethylammonium chlorides |
Occurrence in the environment
Because of their positive charge, the cationic surfactants sorb strongly to the negatively charged surfaces of sludge, soil and sediments. The widespread use and sorption behaviour of cationic surfactants implies that these substances are expected to be present in many environmental compartments. Particular attention was paid to the presence of ditallowdimethylammonium chloride (DTDMAC) in surface waters of major rivers in the Netherlands, where DTDMAC was found at 2 to 34 µg/l (Leeuwen et al. 1992). On the basis of an environmental risk evaluation of DTDMAC, the authorities and the detergent industry in several countries agreed on a voluntary substitution of DTDMAC and the structurally related distearyldimethylammonium chloride (DSDMAC) with readily biodegradable alternatives. During the ninetees quaternary ammonium salts containing ester groups have replaced traditional cationic surfactants in fabric softeners.
5.1 Alkyltrimethylammonium salts
Alkyltrimethylammonium chlorides (ATMAC) and, to a minor extent, alkyltrimethylammonium bromides (ATMAB) are primarily used in cosmetic products including hair conditioners, hair dyes and colors, and other hair and personal care preparations. The hydrophobic alkyl chains of ATMAC and ATMAB are normally linear. These surfactants have the structure:
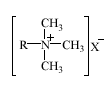
The alkyl chain, R, usually contains 12-18 carbon atoms, and the counter-ion, X-, may be either Cl- or Br-.
5.1.1 Environmental fate
Biodegradation pathways
Very little is known about biodegradation pathways of alkyltrimethylammonium salts. Two potential points of attack were proposed by Macrell and Walker (1978, cited in Ginkel 1995): The degradation may either be initiated by a fission of the C-N bond in which the alkyl chain or a methyl group is cleaved from a tertiary amine, or by an w -oxidation in which the far end of the alkyl chain is first oxidized to a carboxylic acid. Biodegradation can then proceed via b -oxidation. Studies with a Xanthomonas sp. capable of biodegrading C10 ATMAC support both degradation mechanisms as 9-carboxynonyl- and 7-carboxyheptyltrimethyl-ammonium chloride were detected during the growth of the organism on this quaternary ammonium compound (Dean-Raymond and Alexander 1977, cited in Ginkel 1995).
Aerobic biodegradability
Test methods in which the biodegradability is determined by analyses of parent substrate concentration (primary biodegradation) or dissolved organic carbon are less applicable for cationic surfactants because of the strong sorption of these substances. However, the ultimate biodegradability of ATMAC has been examined in several standard biodegradation tests by measuring the oxygen uptake or the evolution of carbon dioxide. The review of Ginkel (1995) cites a number of studies indicating that the recalcitrance of ATMAC in screening tests increases with increasing alkyl chain length. E.g., the studies of Masuda et al. (1976; cited in Ginkel 1995) using the MITI test showed that the biodegradability of various ATMAC during 10 days was 73% of ThOD for C8, 63% for C10, 59% for C12, 35% for C14, and 0% for C16 and C18. These data show that ATMAC can be ultimately degraded in aerobic screening tests. However, information on the inoculum used in the tests is lacking, and, therefore, it is difficult to verify whether or not the OECD criteria for ready biodegradability were fulfilled. During the present study a ready biodegradability test was conducted with C16 ATMAC which was added at 10 mg/l. The results of this test showed that 40% of ThOD was reached during 28 days without acclimation of the inoculum (Table 5.1; Appendix). The bacterial toxicity of especially the longer chained ATMAC may be mitigated in the presence of equimolar amounts of anionic surfactants. Several studies have shown that ATMAC may be extensively mineralized when complexated with the anionic surfactant LAS. E.g., Games et al. (1982) showed that C18 ATMAC at 20 mg/l inhibited the endogenous CO2 production in a SCAS test, and thereby biodegradation was precluded, whereas a mineralization corresponding to 81% of ThCO2 was attained during 25 days in a mixture of C18 ATMAC and LAS (both added at 20 mg/l). Due to the bacterial toxicity and sorptive properties of cationic surfactants, results from screening tests may underestimate the biodegradation potential in the aquatic environment. Rapid and extensive mineralization was observed when 14C-labelled C18 ATMAC was added to the SCAS system at initial levels of 0.1 and 1.0 mg/l (Games et al. 1982; Table 5.1). Another study with 14C-labelled C18 ATMAC (10 µg/l) has demonstrated an extensive mineralization in river water as indicated by the evolution of 14CO2 which corresponded to more than 60% and 75% of the added 14C after 7 and 21 days, respectively (Boethling 1984; Table 5.1). The rapid transformation, which may occur in the environment, can also be illustrated by the half-life of C18 ATMAC which was calculated to 2.2 days in acclimated river water (Larson 1983, cited in Ginkel 1995). The biodegradation routes of alkyltrimethylammonium salts which were outlined above do not indicate that recalcitrant metabolites are formed. This is in accordance with the study of the fate of radiolabelled C18 ATMAC by Games et al. (1982). Using mass balance calculations these authors suggested that no metabolites with appreciable half-lives were formed from the degradation of C18 ATMAC.
Table 5.1
Ultimate aerobic biodegradability of alkyltrimethylammonium chlorides.
ATMAC |
Test |
Result |
Reference |
C16 |
Manometric respirometry test, 10 mg/l; 28 d |
40% ThOD |
This study (Appendix; Table A2; Figure A2) |
C18 |
CO2 evolution screening test, 20 mg/l; 25 d |
Inhibition |
Games et al. 1982 |
C18 |
CO2 evolution screening test, ATMAC + LAS (both 20 mg/l); 25 d |
81% ThCO2 |
Games et al. 1982 |
C18 |
14CO2 test, river water, 10 µg/l; 7 d/21 d |
> 60/75% 14CO2 |
Boethling 1984 |
C18 (14CH3 labelled) |
Unacclimated SCAS system, 1,000 mg SS/l, 0.1 mg/l; 172 h |
88% 14CO2 |
Games et al. 1982 |
C18 (14C1 labelled) |
Unacclimated SCAS system, 1,000 mg SS/l, 0.1 mg/l; 172 h |
67% 14CO2 |
Games et al. 1982 |
Anaerobic biodegradability
Although cationic surfactants will sorb onto sludge particles and eventually reach the digester during the treatment of wastewater sludge, there is very limited information about the biodegradability of these compounds under anoxic conditions. It has been demonstrated, however, that the concentration of quaternary ammonium salts does not decrease, or only slightly decrease, in an anaerobic digester (Janicke and Hilge 1979, cited in Ginkel 1995). The anaerobic biodegradability of C16 ATMAC was examined in the present study by using the ISO 11734 screening test, but the applied test concentration of 14.0 mg C/l was toxic to the anaerobic bacteria as seen from the negative net biogas production throughout the test period of 56 days (Appendix; Table A13, Figure A13).
Bioaccumulation
Bioaccumulation studies with ATMAC have been performed with fathead minnow (Pimephales promelas) by using 14C-labelled model compounds (Tolls et al. 1994). The radiolabelling technique does not allow a distinction between the parent compound and their metabolites formed and, hence, the term concentration ratio (CR) was used instead of BCF which normally refers to the intact parent compound. The relatively few data indicate that the bioconcentration of ATMAC are hydrophobicity dependent as the CRs were 2.4 for C8, 35 for C12, and 1,962 for C16-18 (Versteeg and Shorter 1992, cited in Tolls et al. 1994). The high CR of 1,962 for C16-18 ATMAC may represent both the intact surfactant and its metabolites, and the CR may at least partially be due to inter-experimental variation. Although more experiments are needed to understand the bioconcentration of cationic surfactants, the possibility of variation between experiments is indicated by the fact that the CR for a C(18)2 dialkyldimethylammonium chloride was determined to 104 (Versteeg and Shorter 1992, cited in Tolls et al. 1994).
5.1.2 Effects on the aquatic environment
Algae
Algae constitute a group of organisms which appears to be very sensitive to cationic surfactants. The toxicity of ATMAB and ATMAC to algae is characterized by EC50 values below 1 mg/l (Table 5.2).
Table 5.2
Effects of alkyltrimethylammonium salts to algae.
Species |
Surfactant |
EC50 (mg/l) |
Duration |
Reference |
Selenastrum capricornutum |
C16 ATMAB |
0.09 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
C16 ATMAB |
« 2.5A |
21 d |
Nyberg 1988 |
Microcystis aeruginosa |
C16 ATMAB |
0.03 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
C12 ATMAC |
0.19 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
C12 ATMAC |
0.12 |
96 h |
Lewis and Hamm 1986 |
Navicula pelliculosa |
C12 ATMAC |
0.20 |
96 h |
Lewis and Hamm 1986 |
Dunaliella sp. |
C16-18 ATMAC |
0.38 (0.33-0.45)B |
24 h |
Utsunomiya et al. 1997 |
Chlorella pyrenidosa |
C16-18 ATMAC |
0.28 (0.22-0.26)B |
96 h |
Utsunomiya et al. 1997 |
A
No living cells were observed in the cultures receiving 2.5 mg/l.B 95% confidence limits.
Invertebrates and fish
ATMAC are acutely toxic to aquatic invertebrates as indicated by EC/LC50 values below 1 mg/l for alkyl chain lengths of C16 (Table 5.3). Belanger et al. (1993) exposed artificial stream mesocosms housing the freshwater clam Corbicula fluminea with C12 ATMAC. Minor and transient effects on length gain were observed at 43 m g/l during weeks 2-4 and 6-7, but these effects were not evident at the end of the experiment after 8 weeks. One study with the species Idus melatonus indicates that some ATMAC are also toxic to fish (Boethling and Lynch 1992; Table 5.3).
Table 5.3
Effects of alkyltrimethylammonium chlorides to invertebrates and fish.
Species |
Surfactant |
EC50/LC50 (mg/l) |
Duration |
Reference |
Crustacean |
ATMAC C |
1.2-5.8 |
- |
Boethling and Lynch 1992 |
Crustacean |
C16 ATMAC |
0.1 |
48 h |
Lewis and Suprenant 1983 |
Flatworm |
C16 ATMAC |
0.68 |
48 h |
Lewis and Suprenant 1983 |
Oligochaete |
C16 ATMAC |
0.22 |
48 h |
Lewis and Suprenant 1983 |
Bivalve |
C12 ATMAC |
LOEC:0.18-0.24 A |
56 d |
Belanger et al. 1993 |
Water snail |
ATMAC C |
0.73-23 |
- |
Boethling and Lynch 1992 |
Fish, golden orfe |
ATMAC C |
0.36-8.6 |
- |
Boethling and Lynch 1992 |
A
Effect concentration based on measured concentrations.B 95% confidence limits.
C The ranges include tests with C12, C14, C16, C18, and C20-22.
5.1.3 Effects on human health
Toxicokinetics and acute toxicity
The few available absorption studies conducted with cationic surfactants indicate that absorption occurs in small amounts through the skin (Bartnik and Wingen 1979; SFT 1991). Percutaneous absorption of radiolabelled C12 ATMAB in 3% aqueous solution (applied to an 8 cm2 area with occlusion) in the rat was low and corresponded to 0.6% of the applied 14C activity in 72 hours. Most of the absorbed surfactant was excreted in the urine, i.e. 0.35% of the applied 14C activity within the first 24 hours, whereas 13.2% remained on the skin after rinsing. Cutaneous application of the surfactant without rinsing resulted in a greater degree of percutaneous absorption (3.15%) in 48 hours. In the rat elimination after parenteral administration was rapid and was effected primarily via the urine, - more than 80% of the radioactivity was eliminated within 24 hours of application (Bartnik and Wingen 1979).
About 80% of the 14C activity was found in the gastrointestinal tract 8 hours after oral administration of 14C-labelled C16 ATMAB . Only small amounts of the applied radioactivity were found in the urine and in the blood plasma. This indicates poor intestinal absorption. Similar small amounts of 14C were found in the liver, kidneys, spleen, heart, lungs and skeletal muscles. Within 3 days of ingestion, 92% of the administrated radioactivity had been excreted in the faeces and 1% in the urine. No appreciable enterohepatic circulation of the radioactivity was found (Isomaa 1975).
The acute oral toxicity of alkyltrimethylammonium salts (Table 5.4) is somewhat higher than the toxicity of anionic and nonionic surfactants. This may be due to the strongly irritating effect which cationic surfactants exhibit on the mucous membrane of the gastrointestinal tract (SFT 1991). Cationic surfactants are generally about 10 times more toxic when administrated by the intravenous route compared to oral administration (Falbe 1986; SFT 1991).
Table 5.4
Acute toxicity (LD50) after oral administration of alkyl trimethylammonium salts.
Surfactant |
Species |
LD50 (mg/kg body weight) |
Reference |
C16 ATMAB |
Rat |
1,000 |
Richardson 1992-1994 |
C16 ATMAC |
Rat |
410 |
Richardson 1992-1994 |
C12 ATMAC |
Rat |
250-300 |
Kirk-Otmer 1994 |
C18 ATMAC |
Rat |
1,000 |
Kirk-Otmer 1994 |
C18 ATMAC |
Mouse |
633 |
CIRP 1997 |
C16-18 ATMAC |
Rat |
> 500 |
Kirk-Otmer 1994 |
Skin and eye irritation
Skin irritation depends on surfactant concentration. Regardless of the structure, cationic surfactants lead to serious destruction of the skin at high concentrations. Solutions of approximately 0.1% are rarely irritating, whereas irritation is usually pronounced at concentrations between 1.0 and 10.0% surfactant (CIRP 1997). C16 ATMAC was severely irritating to rabbit skin in a concentration of 2.5%. The surfactant was applied to intact and abraded sites and scored after 34 hours. Then the skin was rinsed and then scored again after 48 hours. The erythema and Eschar Index was 3.75 (maximum 4) and the edema Index was 2.0 (maximum 4) (CIRP 1997).
With regard to eye irritation, cationic surfactants are the most irritating of the surfactants (Bartnik and Wingen 1979; SFT 1991). The longer chained alkyltrimethylammonium salts are less irritating to the rabbit eye than the shorter alkyl chain homologues (CIRP 1997). C10 ATMAB, C12 ATMAB, and C16 ATMAC were tested in concentrations between 0.1 and 1.0% in water and were found to be significantly irritating or injurious to the rabbit eye. A 5% solution of C18 ATMAC was instilled into the eyes of guinea pigs, and this concentration was very irritating with a total PII (The Primary Irritation Index) score of 96 (maximum 110) (Bracher et al. 1987).
A homologous series of ATMAB produced very little swelling of the stratum corneum and some homologues produced a shrinkage of the stratum corneum after prolonged exposure (Jungerman 1970; Putterman 1977; Tupker 1990).
Many proteins in the skin are considerably more resistant to the denaturating effects of cationic surfactants compared to those of anionic surfactants. As cationic surfactants frequently have a lower critical micelle concentration than the anionic surfactants, a saturation of the surfactant/protein complex is prevented by the formation of micelles (SFT 1991). Compared to a representative anionic surfactant, the cooperative binding with subsequent protein denaturation requires about a tenfold higher concentration of a cationic surfactant. Contrary to the irreversible denaturating effect of sodium dodecyl sulfate (C12 AS), the adverse effects of some cationic surfactants on proteins may be reversible (Falbe 1986). Cationic surfactants can interact with proteins or peptides by polar and hydrophobic binding. Polar interactions result in electrostatic bonds between the negatively charged groups of the protein molecule and the positively charged surfactant molecule. For example, the enzyme, glucose oxidase, is deactivated by C16 ATMAB through the formation of an ion pair between the cationic surfactant and the anionic amino acid side-chain of the enzyme molecule (Falbe 1986).
Sensitization
A repeated insult patch test of C16 ATMAC was conducted with 114 volunteers. Seventeen days after the last induction of 0.25% surfactant, a challenge patch of 0.25% was applied. No sensitization was observed (CIRP 1997).
Subchronic/Chronic toxicity
C16 ATMAB was administered at concentrations of 10, 20, and 45 mg/kg/day via the drinking water to rats for one year. The only effect observed was a decrease in body weight gain in the 45 mg/day dose group (Isomaa et al. 1976).
Reproductuive toxicology
No embryo toxic effects were seen, when C18 ATMAC was applied dermally to pregnant rats during the period of major organogenesis (day 6-15 of gestation). The concentrations of C18 ATMAC were 0.9, 1.5 and 2.5%. There was no increase in the incidence of fetal malformations (Palmer et al. 1983). C16 ATMAB was not teratogenic in rats after oral doses. Mild embryonic effects were observed with 50 mg/kg/day, but these effects were attributed to maternal toxicity rather than to a primary embryonic effect. Lower doses of C16 ATMAB showed no embryo toxic or teratogenic effects (CIRP 1997).
Mutagenicity
C16 ATMAC was studied in in vitro short-term tests to detect potential mutagenic effects. Cultures of Syrian golden hamster embryo cells were used for an in vitro bioassay. No in vitro transformation of hamster embryo cells was induced, and C16 ATMAC was not mutagenic in Salmonella typhimurium (Inoue and Sunakawa 1980). No mutagenic effects or genetic damages were indicated in a survey of nine short-term genotoxicity tests with C16 and C18 ATMAC (Yam et al. 1984).
Classification
Most undiluted cationic surfactants satisfy the criteria for classification as Harmful (Xn) with R22 and as Irritant (Xi) for skin and eyes with R38 and R41. In addition, certain surfactants will satisfy the criteria for classification as Corrosive with R34 in addition to the acute toxicity (SFT 1991).
According to CESIO, C8-18 ATMAC (i.e., lauryl, coco, soya, and tallow) are classified as Corrosive (C ) with the risk phrases R22 (Harmful if swallowed) and R34 (Causes burns). C16 ATMAC is classified as Harmful (Xn) with the risk phrases R22 (Harmful if swallowed), R38 (Irritating to skin), and R41 (Risk of serious damage to eyes). C20-22 ATMAC are classified as Irritant (Xi) with R36/38 (Irritating to eyes and skin) (CESIO 2000).
The maximum allowed concentration of C12-22 alkyltrimethylammonium salts (bromide or chloride) in cosmetics is 0.1% (Cosmetic Directive 2000).
5.2 Dialkyldimethylammonium salts
Dialkyldimethylammonium chlorides (DADMAC) are used as antistatic agents in cosmetic products including hair conditioners and hair coloring preparations. Furthermore, DADMAC are used as biocides in industrial cleaning agents and, to a minor extent, all purpose household cleaning agents. The alkyl chains of DADMAC are normally linear, although DADMAC containing at least one branched alkyl chain are also used. The general structure of DADMAC is indicated below.
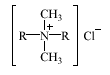
The alkyl chain, R, usually contains 10-16 carbon atoms. The length of the alkyl chains of specific structures is indicated by, e.g., C(12)2 for a DADMAC with two C12 alkyl chains.
5.2.1 Environmental fate
Aerobic biodegradability
The ultimate biodegradability of DADMAC has been examined in several standard biodegradation tests. As for ATMAC, the recalcitrance of DADMAC in screening tests increases with increasing alkyl chain length. This is particularly evident from the studies of Masuda et al. (1976; cited in Ginkel 1995) which indicated that the biodegradability in the MITI test of various DADMAC was 50% of ThOD for C(10)2 and 0% for alkyl chain lengths in the range of C(12)2 to C(18)2. The duration of these tests was 10 days. As also noted for ATMAC, the description of the studies of Masuda et al. does not include information on the inoculum used in the tests. DADMAC with branched alkyl chain(s) like, e.g., decylisononyldimethylammonium chloride are expected to degrade more slowly than similar homologous with linear alkyl chains. Studies by Ginkel et al. (2000) show that DADMAC were transformed in laboratory column experiments in which a slow release of the test compounds were ensured by pre-sorption of the quaternary ammonium salts onto a silica gel. Complete removal of C(10)2 DADMAC, as indicated by HPLC analyses of column effluents, were obtained within 4 days after inoculation of the columns with a pure culture of a bacterium which was able to utilize C(10)2 DADMAC for growth (Table 5.5). In a similar experiment, the same pure culture transformed C(18)2 DADMAC completely after approximately 8 days. A C(16-18)2 DADMAC (ditallow hydrogenated) was transformed in columns inoculated with river water which indicates that microorganisms capable of a primary degradation of DADMAC are common (Ginkel et al. 2000). These results indicate that the poor biodegradability in standard screening tests is not necessarily due to an inherent recalcitrance of DADMAC as other factors like, e.g., toxicity and a slow desorption of the cationic surfactant from surfaces may limit biodegradation. Studies in which 14C-labelled C(16-18)2 DADMAC (ditallow) was added to semi-batch reactors at 2.1 mg/l as a complex with LAS confirm that the entire DADMAC molecule can be ultimately biodegraded. In the reactors, the 14CO2 recovered from mineralization of three radiolabelled forms of C(16-18)2 DADMAC, i.e. [14C]methyl-, [14C]C1-alkyl-, and [14C]uniform-C-labelled, corresponded to between 22 and 53% of the added 14C after 39 days, whereas the primary biodegradation in the same period was somewhat higher, i.e. 59-81% of the initial level (Sullivan 1983). The data in Table 5.5 show that the methyl groups bound to the quaternary nitrogen were more susceptible to biodegradation than the carbons in the alkyl chains. A comparison between the biodegradation of DADMAC (Sullivan 1983) with the C18 ATMAC degradation in the studies of Games et al. (1982) indicates that DADMAC are degraded at a considerably slower rate than ATMAC.
Table 5.5
Ultimate and primary biodegradability of dialkyldimethylammonium chlorides under aerobic
conditions.
DADMAC |
Test |
Result |
Reference |
Ditallow |
Closed bottle test, 283 d |
68% ThOD |
Ginkel 1995 |
Dioctadecyl |
Sturm test, 33 d |
4% ThCO2 |
Ginkel 1995 |
Ditallow, C(16-18)2 [14C]methyl |
Semi-batch reactor, 39 d |
40; 53% 14CO2 |
Sullivan 1983 |
Ditallow, C(16-18)2 |
Semi-batch reactor, 39 d |
31% 14CO2 |
Sullivan 1983 |
Ditallow, C(16-18)2 |
Semi-batch reactor, 39 d |
22; 31% 14CO2 |
Sullivan 1983 |
Didecyl |
Silica gel column, pure culture; 4 d |
100% removal |
Ginkel et al. 2000 |
Dioctadecyl |
Silica gel column, pure culture; 8 d |
100% removal |
Ginkel et al. 2000 |
Ditallow hydrogenated |
Silica gel column, river water; 14 d |
Removal of parent substrate; extent not stated in reference |
Ginkel et al. 2000 |
A short-chained C(8)2 DADMAC was ultimately biodegraded at a concentration of
0.5 mg/l in acclimated river water. The half-lives calculated from the carbon dioxide
produced were 4.9 days in the presence of sediment and 13.8 days without sediment (Larson
1983; Larson and Vashon 1983; both cited in Ginkel 1995).
Anaerobic biodegradability
The information on the biodegradability of cationic surfactants under anoxic conditions is scarce. One study has demonstrated that the concentration of quaternary ammonium salts did not decrease, or only slightly decreased, in an anaerobic digester (Janicke and Hilge 1979, cited in Ginkel 1995).
Bioaccumulation
The bioconcentration of DADMAC has been investigated in studies with bluegill sunfish (Lepomis macrochirus) and fathead minnow (Pimephales promelas). As described for ATMAC in Section 5.1.1 the term CR was used to indicate the bioconcentration which was determined by use of 14C-labelled model compounds. The CR was determined to 32 for C(16-18)2 DADMAC (Lepomis macrochirus) and 104 for C(18)2 DADMAC (Pimephales promelas) (Tolls et al. 1994).
5.2.2 Effects on the aquatic environment
Algae
Algae are very sensitive to dialkyldimethylammonium salts as also noted for the alkyltrimethylammonium salts. The toxicity of DADMAC and DADMAMS to algae is characterized by EC50 values below 1 mg/l (Table 5.6).
Table 5.6
Effects of dialkyldimethylammonium salts to algae.
Species |
Surfactant |
EC50 (mg/l) |
Duration |
Reference |
Dunaliella sp. |
DADMAC, ditallow C(16-18)2 |
18 (13-24)A |
24 h |
Utsunomiya et al. 1997 |
Chlorella pyrenidosa |
DADMAC, ditallow C(16-18)2 |
6.0 (5.5-6.5)A |
96 h |
Utsunomiya et al. 1997 |
Selenastrum capricornutum |
DADMAC, ditallow C(16-18)2 |
0.06 |
96 h |
Lewis and Hamm 1986 |
Selenastrum capricornutum |
DADMAC, ditallow C(16-18)2 |
0.23 B (0.16-0.32)A |
120 h |
Lewis and Wee 1983 |
Selenastrum capricornutum |
DADMAMS, ditallow C(16-18)2 |
0.1-0.5 B |
120 h |
Lewis and Wee 1983 |
Microcystis aeruginosa |
DADMAC, ditallow C(16-18)2 |
0.05 |
96 h |
Lewis and Hamm 1986 |
Microcystis aeruginosa |
DADMAMS, ditallow C(16-18)2 |
0.1 B |
120 h |
Lewis and Wee 1983 |
Navicula pelliculosa |
DADMAC, ditallow C(16-18)2 |
0.07 |
96 h |
Lewis and Hamm 1986 |
A
95% confidence limits.B Algistatic concentration, i.e. the concentration that inhibits growth, but logarithmic growth will resume, when the algae are resuspended in fresh medium without test substance.
Invertebrates and fish
DADMAC with alkyl chains consisting of 16 carbons or more are acutely toxic to aquatic invertebrates and fish as the lowest EC/LC50 values are below 1 mg/l (Tables 5.7-5.8).
Table 5.7
Effects of DADMAC to invertebrates.
Species |
Surfactant |
EC50/LC50(mg/l) |
Duration |
Reference |
Daphnia magna |
Ditallow C(16-18)2 |
0.19 A |
48 h |
Lewis and Wee 1983 |
Daphnia magna |
Ditallow C(16-18)2 |
0.16-1.06 |
48 h |
Kappeler 1982 |
Daphnia magna |
Dioctadecyl C(18)2 |
0.16 A |
48 h |
Lewis and Wee 1983 |
Ceriodaphnia dubia |
Ditallow C(16-18)2 |
0.54 A |
48 h |
Taylor 1984 |
Mysidopsis bahia |
Ditallow C(16-18)2 |
0.22 A |
96 h |
Lewis and Wee 1983 |
Chironomus riparius |
Ditallow C(16-18)2 |
9.2 (8.1-11)B NOEC: 1.34 |
96 h |
Roghair et al. 1992 |
Lymnaea stagnalis |
Ditallow |
18 |
96 h |
Roghair et al. 1992 |
A
Effect concentration based on measured concentrations.B 95% confidence limits.
Table 5.8
Effects of dialkyldimethyl ammonium salts to fish.
Species |
Surfactant |
LC50 (mg/l) |
Duration |
Reference |
Bluegill sunfish (Lepomis macrochirus) |
DADMAC, ditallow |
0.62 A |
96 h |
Lewis and Wee 1983 |
Stickleback |
DADMAC, ditallow |
4.5 |
96 h |
Roghair et al. 1992 |
Bluegill sunfish |
DADMAMS, ditallow C(16-18)2 |
1.23 A |
96 h |
Lewis and Wee 1983 |
Bluegill sunfish |
DADMAC, dioctadecyl |
1.04 A |
96 h |
Lewis and Wee 1983 |
A
Effect concentration based on measured concentrations.B 95% confidence limits.
5.2.3 Effects on human health
No specific data describing the health effects of dialkyldimethylammonium salts were obtained. However, many of the properties described for alkyltrimethylammonium salts also apply to dialkyldimethylammonium salts, although these are generally less irritating than the corresponding alkyltrimethylammonium salts (CIRP 1997).
5.3 Alkyldimethylbenzylammonium salts
Alkyldimethylbenzylammonium chlorides (ADMBAC) and bromides (ADMBAB) are used in cosmetic products including hair conditioners and hair coloring preparations. Besides being surfactants and antistatic agents, the alkyldimethylbenzylammonium compounds function as biocides in various cosmetic and detergent products. The biocidal properties are utilized, when ADMBAC are added to all-purpose or specialized cleaning agents.
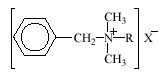
The linear alkyl chain, R, usually contains 8 to 18 carbons, and the counter-ion, X-, may be either Cl- or Br-.
5.3.1 Environmental fate
Biodegradation patyways
The knowledge about the biodegradation pathways of alkyldimethylbenzylammonium salts is very scarce. A qualitative analysis of the metabolites that were formed in pilot activated sludge plants showed that benzoate, acetate, and tetradecyldimethyl amine were formed during degradation of C14 ADMBAC (Fenger et al. 1973). The average degradation of C14 ADMBAC in this study was 73% of the initial concentration during 36 days (Table 5.9). The identified metabolites indicate that ADMBAC is degraded via a cleavage of the bond linking the benzene group to the alkyldimethylammonium.
Aerobic biodegradability
The aerobic biodegradability of ADMBAC has been examined in various standard screening tests. These tests suffer from methodological problems with toxicity and sorption related to the behaviour of cationic surfactants. As for ATMAC and DADMAC, the recalcitrance of ADMBAC in screening tests generally increases with increasing alkyl chain length. The studies of Masuda et al. (1976; cited in Ginkel 1995) indicated that the biodegradability in the MITI test of various ADMBAC was 79% of ThOD for C8, 95% for C10, 89% for C12, 83% for C14, 5% for C16 and 0% for C18 during 10 days of incubation. However, information on the inoculum used by Masuda et al., which is important to evaluate these results, was not reported by Ginkel (1995). A closed bottle test with C12-14 ADMBAC, using a secondary effluent inoculum and a test substance concentration of 1.5 mg/l, showed that only 8% of ThOD was attained during 28 days. Parallel vessels with C12-14 ADMBAC and sodium benzoate revealed that the applied concentration of the test substance inhibited the inoculum by only 16% which indicates that toxicity alone does not explain the poor biodegradability of C12-14 ADMBAC (Madsen et al. 1994). Gerike and Gode (1990) reported 83% ultimate degradation of C12 ADMBAC, as indicated by DOC removal, in a coupled units test (Table 5.9). However, as noted previously, DOC analyses are less applicable for cationic surfactants and results relying on this parameter should therefore be evaluated with caution. Alkyldimethylbenzylammonium salts are clearly better degradable than DADMAC which is particularly evident when comparing the results of the MITI tests by Masuda et al. (1976; cited in Ginkel 1995). The results of Masuda et al. indicate that extensive ultimate biodegradation of ADMBAC (C8 to C14) may occur, and that these surfactants will probably biodegrade as rapidly as ATMAC (see Table 5.1) when present at environmentally realistic concentrations. However, studies with low concentrations of 14C-labelled ADMBAC would improve the basis for evaluating the biodegradability of these substances.
Table 5.9
Ultimate and primary aerobic biodegradability of ADMBAC.
ADMBAC |
Test |
Result |
Reference |
C12 |
CAS test |
83% DOC A |
Gerike and Gode 1990 |
C12 |
CAS test |
96% loss of disulfine blue active substances (primary degradation) 54% DOC A |
Swisher 1987 |
C12-14 |
Closed bottle test, OECD 301D, 1.5 mg/l, 28 d |
8% ThOD |
Madsen et al. 1994 |
C14 |
Activated sludge pilot plants, 20 mg/l, 36 d |
63-72% loss of parent |
Fenger et al. 1973 |
A
Sorbed DOC, if any, could probably not be differentiated from the sludge itself.Anarobic biodegradability
Only limited information exists on the biodegradability of cationic surfactants under anoxic conditions. A study by Janicke and Hilge (1979, cited in Ginkel 1995) has demonstrated that the concentration of quaternary ammonium salts did not decrease, or only decreased slightly, in an anaerobic digester.
5.3.2 Effects on the aquatic environment
ADMBAC are very toxic to aquatic organisms as also noted for the alkyltrimethyl ammonium and dialkyldimethylammonium salts. Some of the available data on the acute aquatic toxicity (EC/LC50) are below 1 mg/l (e.g. for the green algae Chlorella pyrenidosa), but EC/LC50 values between 1 and 10 mg/l are also observed (Table 5.10).
Table 5.10
Aquatic toxicity of ADMBAC.
Species |
Surfactant |
EC50/LC50 |
Duration |
Reference |
Green alga |
C12-14 |
1.8 |
24 h |
Utsunomiya et al. 1997 |
Green alga |
C12-14 |
0.67 |
96 h |
Utsunomiya et al. 1997 |
Golden orfe |
C12 |
LC0: 3.5 |
- |
Boethling and Lynch 1992 |
Bluegill sunfish |
Hyamine 3500 |
0.5 |
- |
Boethling and Lynch 1992 |
Goldfish |
Hyamine 3500 |
2.0 |
- |
Boethling and Lynch 1992 |
5.3.3 Effects on human health
Toxicokinetics and acute toxicity
No specific toxicokinetic studies were identified for ADMBAC, but the absorption of these surfactants through the skin is anticipated to be low as observed for the alkyltrimethylammonium salts (Section 5.1.3). Different homologues of ADMBAC showed a moderate acute toxicity in experiments with rats and mice (Table 5.11).
Table 5.11
Acute toxicity (LD50) of ADMBAC.
Surfactant |
Species |
Application |
LD50 (mg/ kg body weight) |
Reference |
ADMBAC |
Rat |
Oral |
300 |
Lewis 1996 |
ADMBAC |
Rat |
Oral |
280-445 |
BIBRA 1989 |
C12-18 ADMBAC |
Rat |
Oral |
525 |
CIRP 1989 |
C14-18 ADMBAC |
Mouse |
Oral |
150-340 |
BIBRA 1989 |
C14-18 ADMBAC |
Rat |
Dermal |
1,420 |
Lewis 1996 |
The relationship between alkyl chain length and the acute toxicity of various ADMBAC
homologues (C8 to C19) has been studied in mice. The studies
indicated that chain lengths above C16 had a markedly lower acute toxicity and
that even-numbered alkyl chain homologues appeared to be less toxic than odd-numbered
carbon chains. It was suggested that the decrease in toxicity above C16 was due
to a decreased water-solubility (Zeiger and Anderson 1987; CIRP 1989).
Dermal and eye irritation
ADMBAC is a skin irritant in animals at concentrations above 0.1% (CIRP 1989). A non-specified ADMBAC caused skin irritation and minor to moderate eye irritation at 0.625 and 1.25% concentrations (Skydsgaard and Dideriksen 1991). Inflammation of the eye and deterioration of vision occurred 3 days after change of soaking solution for a soft contact lens to a solution containing C8-18 ADMBAC (Richardson 1992-1994).
Sensitization
The sensitization potential of ADMBAC has been examined in an experiment including 2,295 patients with suspected allergic contact dermatitis. Some of the patients (5.5%) showed positive reactions after exposure to 0.1% ADMBAC. These results were surprising as ADMBAC was not suspected to be a sensitizer. The high irritating potential of ADMBAC, even at low concentrations, could be an explanation of the observed results as the patch test reactions may have been false positives (Perrenoud et al. 1994). However, another group of 2,806 patients with eczema was patch tested with 0.1% ADMBAC, and 2.13% of these patients appeared to be sensitized (Camarasa 1979). Skin sensitization was noted in patients patch tested with ADMBAC in aqueous solutions at 0.07 to 0.1% surfactant. However, there was no incidence of skin sensitization in a population of normal individuals tested with 0.1% ADMBAC. This indicates that individuals with diseased skin may be at risk for sensitization to ADMBAC (Afzelius and Thulin 1979; Lovell and Staniforth 1981).
Mutagenicity
C16 ADMBAC did not induce transformation of the cells in an in vitro bioassay for carcinogenesis by using cultures of Syrian golden hamster embryo cells. The mutagenic potential of this surfactant was also examined by using Salmonella typhimurium strains - no mutagenic effects were seen (Inoue and Sunakawa 1980). In other short-term genotoxicity assays (Salmonella/microsome assay) and rec-assay (bacterial DNA repair test) C16 ADMBAC was tested for ability to cause DNA damage in bacteria. None of the data indicated any mutagenic effects (Yam et al. 1984).
Carcinogenicity
Lifetime studies of ADMBAC were conducted in mice and rabbits that were treated with 8.5 to 17% surfactant dissolved in acetone or methanol. ADMBAC was applied repeatedly to the skin and ADMBAC caused ulceration, inflammations and scars in many animals, but no tumours (Steinbäck 1977).
Reproductivity toxocoty
No embryotoxic activity was detected when C18 ADMBAC was applied topically to pregnant rats during the period of major organogenesis (day 6-15) at doses up to 6.6%, which was sufficient to cause adverse maternal reactions (Palmer et al. 1983). Intravaginal instillation of ADMBAC (single doses up to 200 mg/kg) to pregnant rats on day one of the gestation caused abnormal foetal development and embryotoxicity (Buttar 1985).
Classification
ADMBAC are included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC with the following classification:
C8-18 ADMBAC are classified as Harmful ( Xn) with the risk phrases R21/22 (Harmful in contact with skin and if swallowed) and Corrosive (C) with R34 (Causes burns) and (N) with R50 (Very toxic to aquatic organisms).
5.4 Alkyl ester ammonium salts
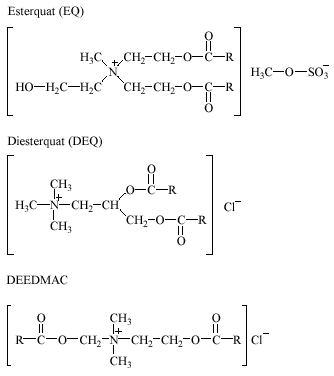
During the last decade alkyl ester ammonium salts have largely replaced the dialkyldimethylammonium salts (e.g. DTDMAC and DSDMAC) in fabric softeners for household use. Alkyl ester ammonium salts are quaternary ammonium compounds containing one, or more often two, weak ester linkages in the molecular structure. This group of cationic surfactants consists of at least three different types of esters: (I) the esterquat (EQ), N-methyl-N,N-bis[2-(C16-18–acyloxy) ethyl]-N-(2-hydroxyethyl) ammonium methosulfate, (II) the diesterquat (DEQ), N,N,N-trimethyl-N-[1,2-di-(C16-18–acyloxy) propyl] ammonium, and (III) the diethyl ester dimethylammonium chloride (DEEDMAC), di-(tallow fatty acid) ester of di-2-hydroxyethyl dimethylammonium chloride.
The structures of alkyl ester ammonium salts are given below.
5.4.1 Environmental fate
Effects of structure on biodegradability
The presence of ester linkages implies that a rapid biodegradation is expected for all alkyl ester ammonium salts described above. The ester linkages are readily attacked by microorganisms, and the cleavage of these linkages results in smaller molecules that are easily biodegraded.
Aerobic biodegradability
The aerobic biodegradability of the poorly water-soluble EQ has been examined under simulated sewage treatment plant conditions in the coupled units test in which more than 90% degradation was found (Puchta et al. 1993). The main metabolite formed from the degradation of the EQ was a tris-(hydroxyethyl) methylammonium methosulfate (MTEA), and since this metabolite is a water-soluble substance with a quaternary structure, further tests for ready biodegradability were carried out with MTEA (Table 5.11). The parent molecules of DEQ and DEEDMAC have been examined in standard OECD screening tests for ready biodegradability. Although these compounds also have a low water-solubility (e.g. 2.8 µg/l for DEQ), both DEQ and DEEDMAC have proven to be readily biodegradable under screening test conditions (Table 5.12).
Table 5.12
Ultimate aerobic biodegradability of alkyl ester ammonium salts.
Compound |
Test |
Result |
Reference |
MTEA |
CO2 evolution test, 28 d |
76-94% ThCO2 |
Puchta et al. 1993 |
DEQ |
CO2 evolution test, 10/20 mg/l, 28 d |
85%; 87% ThCO2 |
Waters et al. 1991 |
DEEDMAC |
CO2 evolution test, 10/20 mg/l, 28 d |
80% ThCO2 |
Giolando et al. 1995 |
The mineralization of 14C-stearyl-, 14C-methyl-, and 14C-dihydroxypropyl-labelled
DEQ in river water attained 94.1, 88.4, and 94.6% of the 14C added during 22
days. The associated mineralization half-lives of DEQ were determined to 0.65-0.70 days,
7.1-7.7 days, and 6.1-6.7 days, respectively, for the various positions of the 14C
(Waters et al. 1991). The mineralization of 14C-labelled DEEDMAC was
examined in activated sludge and river water with sediment (Giolando et al. 1995).
The total accumulated 14CO2 from the mineralization of DEEDMAC
attained 76% and 82% of the added 14C for the batch activated sludge and the
river water die-away test, respectively. The estimated half-lives for the mineralization
of DEEDMAC were 1.0 days in activated sludge and 1.1 days in river water with sediment
(Giolando et al. 1995). The findings in the studies with 14C-labelled
DEQ and DEEDMAC indicate that these compounds will be rapidly and completely biodegraded
in a variety of environmental compartments.
Anaerobic biodegradability
The ultimate anaerobic biodegradability of DEEDMAC has been examined in the ECETOC test (ECETOC 1988). The total gas production from mineralization of DEEDMAC reached 90% of ThGP during 60 days under the methanogenic test conditions (Giolando et al. 1995). No data were found on the anaerobic biodegradation of EQ and DEQ, but due to the structural similarity with DEEDMAC (primarily the ester linkages) EQ and DEQ are assumed to be degraded under anoxic conditions as well.
5.4.2 Effects on the aquatic environment
Alkyl ester ammonium salts generally have an acute aquatic toxicity characterized by EC/LC50 values between 2 and 10 mg/l (Table 5.13). The aquatic toxicity of alkyl ester ammonium salts is markedly lower as compared with other cationic surfactants. A comparison with the EC/LC50 values for ATMAC, DADMAC, and ADMBAC shows that the acute aquatic toxicity of alkyl ester ammonium salts is at least one order of magnitude lower (i.e., EC/LC50 are higher) than the toxicity of the ‘traditional’ quaternary ammonium compounds.
Table 5.13
Effects of alkyl ester ammonium salts to aquatic organisms.
Species |
Surfactant |
EC/LC50 (mg/l) |
Duration |
NOEC(mg/l) |
Reference |
Algae |
Esterquat (EQ) |
|
- |
0.3 |
Puchta et al. 1993 |
Algae |
DEQ |
|
72 h |
1.8 |
Waters et al. 1991 |
Algae |
DEEDMAC |
2.9 |
96 h |
|
Giolando et al. 1995 |
Daphnia |
Esterquat (EQ) |
78 |
21 d-NOEC |
3.0 |
Puchta et al. 1993 |
Daphnia magna |
Diesterquat (DEQ) |
7.7 |
48 h-EC50 |
1.0 |
Waters et al. 1991 |
Daphnia magna |
DEEDMAC |
14.8 |
24 h-EC50 |
1.0 |
Giolando et al. 1995 |
Fish |
Esterquat (EQ) |
3.0 |
14 d-NOEC |
4.0 |
Puchta et al. 1993 |
Rainbow trout |
Diesterquat (DEQ) |
7.0 |
96 h-LC50 |
³ 3.5 |
Waters et al. 1991 |
Zebra fish |
DEEDMAC |
5.2 |
96 h |
|
Giolando et al. 1995 |
Fathead minnow |
DEEDMAC |
|
35 d |
0.68 |
Giolando et al. 1995 |
5.4.3 Effects on human health
Acute toxicity
Rats and mice given oral doses of 5,000 mg of EQ/kg body weight exhibited no symptoms of toxic reactions (Puchta et al. 1993). The LD50 values by oral administration and dermal application of DEQ were more than 5,000 mg/kg body weight in rats and more than 2,000 mg/kg body weight for rabbits, respectively (Waters et al. 1991). These results indicate a very low acute toxicity of alkyl ester ammonium salts.
Skin and eye irritation
Concentrated EQ was found to be irritating to the skin of rabbits after 4 hours of semi-occlusive exposure, but the irritation is reversible (Puchta et al. 1993). DEQ was found to be non-irritant to the skin and eye of rabbits (Waters et al.1991).
Skin sensitization
No sensitization potential of EQ was detected in guinea pigs by use of the maximization method (Puchta et al. 1993). Also DEQ was not sensitizing in a modified Buehler test using guinea pigs (Waters et al. 1991).
Subchronic toxicity
A 90-days feeding study in rats showed no systemic toxic effects after administration of doses of up to 300 mg of EQ/kg body weight and even when the dose was increased to 1,000 mg/kg body weight (Puchta et al. 1993). A 28-day subchronic toxicity test with DEQ showed no apperant adverse effects on rats fed a diet containing up to 1% DEQ (Waters et al. 1991).
Mutagenicity
EQ showed no gene mutation effects in the Ames test and no chromosome mutations in the Micronucleus test (Puchta et al. 1993). No genetic damage after exposure to DEQ was indicated in tests for gene mutation and chromosomal aberration (Waters et al. 1991).