Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products
6. Amphoteric surfactants
Surface-active compounds with both acidic and alkaline properties are known as amphoteric
surfactants. Amphoteric surfactants include two main groups, i.e. betaines and real
amphoteric surfactants based on fatty alkyl imidazolines. The key functional groups in the
chemical structures are the more or less quaternized nitrogen and the carboxylic group.
Betaines are characterized by a fully quaternized nitrogen atom and do not exhibit anionic
properties in alkaline solutions, which means that betaines are present only as
‘zwitterions’. Another group of amphoterics is designated imidazoline
derivatives because of the formation of an intermediate imidazoline structure during the
synthesis of some of these surfactants. This group contains the real amphoteric
surfactants that form cations in acidic solutions, anions in alkaline solutions, and
‘zwitterions’ in mid-pH range solutions. The mid-pH range (isoelectric range) in
which the surfactant has a neutral charge is compound specific and depends on the
alkalinity of the nitrogen atom and the acidity of the carboxylic group (Domsch 1995).
Amphoteric surfactants are used in personal care products (e.g. hair shampoos and
conditioners, liquid soaps, and cleansing lotions) and in all-purpose and industrial
cleaning agents. The total volume of amphoteric surfactants consumed in commercial
products today is relatively small (see Chapter 2), but the consumption of these chemicals
is expected to increase in the future because of the request for milder surfactants.
Besides acting as mild surfactants, the amphoterics may improve the mildness of especially
anionic surfactants. By volume, the most important groups of amphoteric surfactants today
consist of alkylamido betaines and alkyl betaines. The use of alkylamphoacetates in
personal care products is expected to grow in coming years.
6.1 Betaines
Betaines are primarily used in personal care products like, e.g. hair shampoos, liquid soaps, and cleansing lotions. Other applications include all-purpose cleaning agents, hand dishwashing agents, and special textile detergents. All betaines are characterized by a fully quaternized nitrogen. In alkyl betaines, one of the methyl groups in the ‘betaine’ structure (N,N,N-trimethylglycine) is replaced by a linear alkyl chain. A special type of betaines is the hydroxysulfobetaines in which the carboxylic group of alkyl betaine is replaced by sulfonate and a hydroxy-group is inserted in the hydrophilic part of the molecule. In alkylamido betaines, an amide group is inserted as a link between the hydrophobic alkyl chain and the hydrophilic moiety. The most commonly used alkylamido betaine is alkylamidopropyl betaine (e.g., cocoamidopropyl betaine), whereas alkylamidoethyl betaines are used in smaller amounts.
Representative structures of betaines are shown below.
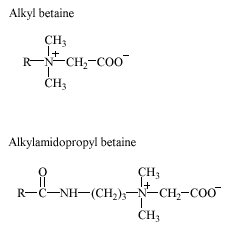
No data were found on the occurrence of betaines in the environment.
6.1.1 Environmental fate
Aerobic biodegradability
The primary biodegradability of betaines approaches 100% as, e.g., the loss of surface-activity attained 100% for C12 alkyl betaine, 98% for cocoamidopropyl betaine, and 96 and 100% for C14-15 hydroxysulfobetaine (Domsch 1995, and references therein). The results from ultimate biodegradability tests of alkyl betaines show some variation with degradation percentages below and above the pass level for ready biodegradability, especially if older data of Fernlay (1978, cited in Domsch 1995) are taken into account. However, both alkyl betaines and cocoalkylamido betaines can be regarded as readily biodegradable on the basis of the data in Table 6.1. The hydroxysulfobetaines are probably not readily biodegradable in standard screening tests as indicated by a biodegradability of 40 and 47% of ThOD in the closed bottle test (Table 6.1).
Table 6.1
Ultimate aerobic biodegradability of betaines.
Compound |
Test |
Result |
Reference |
C12-14 alkyl betaine |
Closed bottle test, 28 d |
63% ThOD |
Madsen et al. 1994 |
C12-18 alkyl betaine |
Closed bottle test, 28 d |
> 60% ThOD |
Brøste 1998 |
Cocoalkyl betaine |
Closed bottle test, 30 d |
> 60% ThOD |
Domsch 1995 |
Cocoalkyl betaine |
Closed bottle test, 30 d |
57% ThOD |
Domsch 1995 |
Cocoalkyl amidopropyl betaine |
Closed bottle test, 30 d |
84% ThOD |
IUCLID 2000 |
Cocoalkyl amidopropyl betaine |
Modified OECD screening test, 28 d |
100% DOC |
IUCLID 2000 |
Cocoalkyl amidopropyl betaine |
Modified OECD screening test |
90-94% DOC |
Domsch 1995 |
C14-15 hydroxysulfo betaine |
Closed bottle test |
40% ThOD |
Domsch 1995 |
Cocoalkyl hydroxysulfo betaine |
Closed bottle test |
47% ThOD |
Domsch 1995 |
Anaerobic biodegradability
The knowledge about the biodegradability of betaines under anoxic conditions is relatively scarce. A search in the literature by Goldschmidt (1993-1994) indicates that sulfate-reducing marine bacteria belonging to the genus Desulfobacterium are able to grow on betaine with the stoichiometric formation of N,N-dimethylglycine (Heijthuijsen and Hansen 1989, cited in Goldschmidt 1993-1994). Another study indicated that betaine was anaerobically degraded to methylamine in sewage sludge at a betaine concentration of 2 g/l and a solids concentration of 3.3 g/l (Gwardys and Nowakowska-Waszczuk 1981, cited in Goldschmidt 1993-1994). The anaerobic biodegradability of cocoamidopropyl betaine was examined in the present study by using the ISO 11734 screening test. Under the methanogenic test conditions, the ultimate biodegradability of cocoamidopropyl betaine attained 45 and 75% of ThGP after 28 and 56 days, respectively, at the applied test concentration of 14.4 mg C/l (Appendix; Table A14, Figure A14).
Bioaccumulation
No experimental data describing the bioaccumulation potential of betaines were found in the literature.
6.1.2 Effects on the aquatic environment
The aquatic toxicity of betaines varies considerably, even within the same species, which is particularly evident by evaluating the EC50 values determined for the green alga Scenedesmus subspicatus. For this species, the EC50 obtained in tests with cocoamidopropyl betaine are between 0.55 and 48 mg/l. The geometric mean of the EC50 obtained for S. subspicatus is 3.1 mg/l, when the values 0.55, 1.84, and 30 mg/l are used (Table 6.2). The EC/LC50 of alkyl and cocoamidopropyl betaines towards crustaceans and fish are between 1 and 100 mg/l.
Table 6.2
Effects of alkyl and alkylamidopropyl betaines to aquatic organisms.
Species |
Surfactant |
EC/LC50 (mg/l) |
Duration |
Reference |
Algae |
C12-14 alkyl betaine |
2.5 |
72 h |
Berol Nobel 1993 |
Algae |
Cocoamidopropyl betaine |
1.84 |
72 h |
IUCLID 2000 |
Algae |
Cocoamidopropyl betaine |
Growth rate: |
96 h |
IUCLID 2000 |
Algae |
Cocoamidopropyl betaine |
Biomass: |
72 h |
Goldschmidt 1993-1994 |
Daphnia magna |
Cocoamidopropyl betaine |
6.5 |
48 h |
IUCLID 2000 |
Daphnia magna |
Cocoamidopropyl betaine |
21.7 |
48 h |
IUCLID 2000 |
Zebra fish |
C12-14 alkyl betaine |
21.9 |
96 h |
Berol Nobel 1993 |
Fish |
C12-18 alkyl betaine |
10-100 |
- |
Brøste 1998 |
Zebra fish |
Cocoamidopropyl betaine |
2.0 |
96 h |
IUCLID 2000 |
6.1.3 Effects on human health
Toxicokinetics and acute toxicity
Amphoteric surfactants are easily absorbed in the intestine and are excreted partly unchanged via the faeces. Metabolization to CO2 and short-chained fatty acids also occur. No tendency to accumulation in the organism or storage of betaines in certain organs has been detected (SFT 1991). Betaines generally have a low acute toxicity. E.g., LD50 values for cocoamidopropylbetain (30% solution) by oral administration have been determined to 4,910 mg/kg body weight in rats (CIRP 1991a).
Skin and eye irritation
Betaines do not carry any net charge, and, therefore, they can only form hydrophobic bonds with proteins in the skin. This may be the explanation for the low protein denaturation potential of betaines as the ion-binding of other surfactants contributes to denaturation. In combination with anionic surfactants a positive synergistic effect with regard to skin compatibility is often found. Compared to a 20% solution of C12 alkyl sulfate (AS; sodium lauryl sulfate) alone, decreased erythema was observed for the combination of 20% C12 AS and 10% cocoamidopropyl betaine one hour after the removal of patches (Dillarstone and Paye 1993). The combination of cocoamidopropyl betaine and C12 AS also reduced swelling of the skin, and generally interactions between amphoterics and AS produce less swelling and result in milder skin reactions (Rhein et al. 1986).
Concentrated betaines are expected to be irritant to skin and eyes. Diluted solutions (3-10%) are not irritant to skin, but they are mildly irritant to the eyes (4.5%) (KEMI 1994).
Solutions containing 7.5% and 10% cocoamidopropyl betaine were not irritating to intact or abraded rabbit skin in a single insult occlusive patch test. The PII (Primary Irritation Index) for the solution was < 0.3 (maximum score is 8). When a 15% solution was tested under occlusive patches for 24 hours by using the same procedure, a PII of 3.5 was achieved and well-defined erythema and edema were observed (CIRP 1991a).
In a Draize test for ocular irritation a concentration of 4.5% cocoamidopropyl betaine produced a slight conjunctival irritation (erythema and swelling of conjunctiva) in unrinsed eyes and a very slight conjunctival irritation in rinsed eyes of rabbits. The surfactant was instilled into the conjunctival sac of the eye. No corneal involvement or iris congestion was seen (CIRP 1991a). The maximum mean irritation scores for eyes of rabbits treated with 30% cocoamidopropyl betaine and left unrinsed were in the range between 26 and 42 (maximum score is 110) (CIRP 1991a).
Sensitization
No evidence of delayed contact hypersensitivity was found in guinea pigs after topically administrated solutions of 10% cocoamidopropyl betaine by using the Magnusson-Kligman maximization test (CIRP 1991a). Various instances of contact allergy to cocoamidopropyl betaine have been reported. In all of the reports it was concluded that the observed skin reactions were due to the presence of 3-dimethylaminopropylamine which is an impurity in cocoamidopropyl betaine. This impurity is an intermediate in the synthesis of alkylamidopropyldimethylamines that are intermediates in the synthesis of the corresponding alkylamido betaines (Angelini et al. 1995, 1996a, 1996b; Armstrong et al. 1999).
Mutagenicity
Cocoamidopropyl betaine was proven to be non-mutagenic to Salmonella typhimurium in the Ames Salmonella/microsome reverse mutation assay (CIRP 1991a). Short-term genotoxicity tests have shown negative results of mutagenicity for lauryl betaine in various strains of Salmonella typhimurium (Yam et al. 1984).
No tests on reproductive toxicity and carcinogenicity were available.
Classification
Betaines are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.
6.2 Imidazoline derivatives
The amphoteric surfactants in this group include structures designated as alkylamphoacetates, alkylamphopropionates, and alkyliminopropionates. These surfactants are usually produced by the reaction of fatty acids or their esters with amines (e.g. aminoethylethanol amine). Alkylamphopropionates may be obtained by the addition of acrylic acid, methyl acrylate, or ethyl acrylate to the reaction product of fatty acids and amines. During the synthesis of most of the surfactants an intermediate imidazoline ring structure may be formed (hence the common name ‘imidazoline derivatives’). The imidazoline ring is probably opened by the influence of hydrolysing conditions and does not appear in the final products (Domsch 1995). Alkylamphoacetates, alkylamphopropionates, and alkyliminopropionates are used in products like hair shampoos, liquid soaps, and shower gels. Other major applications of alkylamphopropionates and alkyliminopropionates include highly acidic and alkaline household cleaning agents. Commercial products may contain complex mixtures of the amphoteric surfactants described in this section. Representative structures are given below.
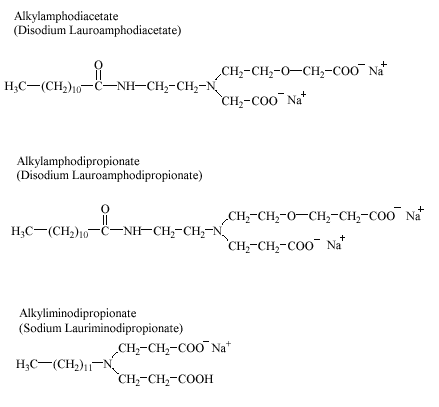
No data were found on the occurrence of these amphoteric surfactants in the environment.
6.2.1 Environmental fate
Aerbic biodegradability
The ultimate aerobic biodegradability of alkylamphodiacetates, alkylamphopropionate, and alkyliminodipropionate complies with the pass levels for ready biodegradability in OECD 301 screening tests (Table 6.3).
Table 6.3
Ultimate aerobic biodegradability of amphoteric imidazoline derivatives.
Compound |
Test |
Result |
Reference |
Cocoamphodiacetate |
Closed bottle test, 30 d |
> 60%; 66% ThOD |
Domsch 1995 |
Cocoamphodiacetate |
Modified OECD screening test |
> 70% DOC |
Domsch 1995 |
C12-18 alkylampho-propionate |
Modified OECD screening test |
79% ThOD |
Domsch 1995 |
C12 alkylimino-dipropionate |
Manometric respirometry test, 28 d |
99% ThOD |
This study (Appendix; Table A3, Figure A3) |
Anaerobic biodegradability
Information on the ultimate anaerobic biodegradability of imidazoline derivatives has not been found in the literature. The anaerobic biodegradability of C12 alkyliminodipropinate (16.4 mg C/l) reached only 2.5% of ThGP during 56 days in the ISO 11734 screening test which was performed in the present study. However, the test substrate concentration inhibited the anaerobic bacteria, and inhibitory effects may have precluded biodegradation (Appendix; Table A15, Figure A15).
Bioaccumulation
No experimental data describing the bioaccumulation potential of alkylamphoacetates, alkylamphopropionates, or alkyliminopropionates were found in the literature.
6.2.2 Effects on the aquatic environment
Acute toxicity
No data describing the aquatic toxicity of the amphoteric surfactants in this group were found in the literature. Because of the variability in the effect concentrations observed for betaines (see Table 6.2), it is not tempting to base the assessment upon structural analogy and betaine aquatic toxicity. Testing of the aquatic toxicity and the subsequent release of data to the open literature should be encouraged as the consumption of these surfactants is expected to increase.
6.2.3 Effects on human health
Alkylamphoacetates and akylamphopropionates have a low acute toxicity after oral administration to rats (Table 6.4).
Table 6.4
Acute toxicity (LD50) of amphoteric surfactants by oral administration.
Surfactant |
Species |
LD50 (g/kg body weight) |
Reference |
Cocoamphoacetate |
Rat |
15.9 – 28 ml |
CIRP 1990 |
Cocoamphodiacetate |
Rat |
> 5.0 – 16.6 |
CIRP 1990 |
Cocoamphopropionate |
Rat |
20.0 ml* |
CIRP 1990 |
Cocoamphodipropionate |
Rat |
> 5.0 – 16.3 |
CIRP 1990 |
* Commercial solution in water, probably 40-50%.
Skin and eye irritation
Generally these amphoteric surfactants do not seem to be irritant to the skin and only to a small extent irritating to the eye (SFT 1991). Some variation in test results have been reported.
Cocoamphodipropionate was found to be non-irritating as a concentration of 7.5-70% (PII = 0), whereas cocoamphopropionate was slightly irritating to rabbit skin at a concentration of 15–16%. Cocoamphodiacetate was non-irritating to slightly irritating at a concentration of 10-12% (CIRP 1990).
A Draize test has shown that cocoamphodipropionate was practically non-irritating to the eye at a concentration of 7.5%, whereas cocoamphopropionate was non-irritating to slightly irritating at 5% and 16%. Cocoamphodiacetate was moderately to severely irritating to the eye at a concentration of 10-12%. Cocoamphoacetate was slightly to severely irritating at 16 to 50% (CIRP 1990).
Sensitization
Cocoamphoacetate and cocoamphopropionate were non-irritating and non-sensitizing in a repeated insult patch test (non-occlusive) involving 141 subjects. The concentration of the surfactants was 10% in distilled water. During induction, each chemical was applied to the back three times per week for three weeks. The challenge phase was initiated 10 to 15 days after application of the final induction patch. Cocoamphoacetate and cocoamphopropionate did not induce sensitization in any of the subjects (CIRP 1990). Cocoamphoacetate was non-sensitizing in guinea pigs when tested in the Magnusson-Kligman maximization test. The tested concentrations for induction and challenge were 25, 50 and 100% (CIRP 1990).
Mutagenicity
Cocoamphodiacetate, cocoamphopropionate, and cocoamphodipropionate were non-mutagenic, when evaluated in the Ames Salmonella/microsome assay using different strains of Salmonella typhimurium (CIRP 1990).
No tests on reproductive toxicity and carcinogenicity were available.
Classification
The amphoteric surfactants described in this section are not included in Annex 1 of list of dangerous substances of Council Directive 67/548/EEC.