Dermal absorption of pesticides - evaluation of variability and prevention
4 Methods to study dermal penetration
During the last decade much attention related to percutaneous penetration has been directed towards standardization and validation of experimental models. In vitro as well as in vivo methods exist, each with their own set of advantages and disadvantages. Which method to use will depend on the research question to be answered, which is probably also one of the reasons why the present OECD guideline for studies on percutaneous penetration accepts several methods. Equally important to the researcher generating experimental data as to the user of this data (e.g. governmental agencies) is a clear appreciation of the pros and cons that each method has. This chapter will describe some of the frequently used experimental methods and their different advantages and limitations.
4.1 In vitro technique
Standardization and validation of different in vitro models have been described by the Percutaneous Penetration Subgroup of EC Dermal Exposure Network (Sartorelli et al., 2000). A recent inter-laboratory comparison of experimental models on percutaneous penetration involving nine European laboratories demonstrated good agreement between data on selected model compounds obtained in the different laboratories, given that comparable experimental procedures were used (van de Sandt et al., 2004).
In vitro methods are used in laboratories all over the world with the intention to assess the penetration characteristics of specific substances. A range of different designs have been developed with the general aim to measure the penetration of agents through the skin membrane into a fluid reservoir. As previously described, several factors influence the permeation of a substance such as solubility, molecular weight and size, penetrant-skin binding, barrier function etc.
In 2004 OECD issued a guideline for testing chemicals by in vitro methods. The standard principles were described for the use of the static diffusion cell on the flow-through system. The test substance is in both methods applied to the surface of the skin which separates the donor and the receptor chambers. The amount of penetrated substance is measured in the receptor fluid as a function of time. Experimental approaches include infinite as well as finite dosing and may also include setups where test substance remains on the skin for a specified time under specified conditions, before removal by an appropriate cleansing procedure. It is important to keep the receptor fluid (and sometimes the donor substance) homogeneous by stirring (OECD, 2004).
4.1.1 Static diffusion cells
In 1975 Franz developed a static diffusion cell which is now one of the most commonly used in vitro systems in the research of skin penetration. The system has a simple design and is inexpensive to use (Figure 7). Human as well as animal skin can be mounted on the metal grid which divides the donor chamber and the receptor chamber. The skin is set placing the dermis in contact with the receptor fluid below. The skin can be either full-thickness or split-thickness skin. The skin thickness will affect the experimental results (van de Sandt et al., 2004) as elaborated under Flow-through system. The receptor chamber of the cell is placed in circulation water in a water bath with a temperature of 37 ºC keeping the temperature at the skin surface at 32° to imitate a real life skin condition as much as possible. The receptor fluid is kept homogenous in concentration and in temperature by a magnetic stirring bar. The fluid in the receptor chamber is manually sampled at predefined time intervals. Any type and any amount of vehicle (that will fit into the donor chamber) may be applied to the skin.
Franz showed an excellent correlation between in vitro and in vivo studies (Franz, 1975).
When testing different substances it is important to be aware of the solubility of the substance. The solubility of a substance influences the sink capacity and is therefore of great importance when it comes to choosing the right sampling frequency and receptor chamber dimension. The size of the receptor chamber determines when the receptor fluid achieves a certain degree of saturation (Brain KR et al., 1998).
The barrier integrity of the skin can be evaluated by capacitance measurement. This value indicates the ability of the skin to separate electrical charge.
Skin samples with a high capacitance are unable to act as capacitors, which means that the skin is damaged. The measurements are carried out at the beginning and at the end of the study to give an accurate evaluation of the skin barrier.

Figure 7: Static diffusion cell.
4.1.2 Flow-through system
Another in vitro model is the flow-through system (Figure 8 and 9). This is a system consisting of multiple cells. The system is developed by Bronaugh and Stewart in 1985 and is excellent for determining the reservoir effect of the skin (Bronaugh & Stewart, 1985a). The flow-through cells can - as well as the static cells - be mounted with animal or human full- or split-thickness skin, which will generate skin barriers of different thickness and as in the static cells the skin thickness will affect the experimental results (van de Sandt et al., 2004). Prolonged lag-times might be expected in experiments using full-thickness skin. The type of skin preparation generating the most valid data is not obvious and probably one of the reasons why OECD accepts the different experimental approaches in their guidelines (OECD, 2000).
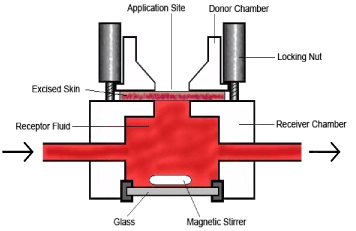
Figure 8: Flow-through cell (Use of picture permitted by Dr. Wilkinson SC, University of Newcastle).
The receptor fluid is in the flow-through cells continuously replaced and collected every hour to imitate an in vivo situation where the blood circulation removes the transdermal penetration substances. This has an additional benefit when dealing with substances with low solubility in the receptor medium and the sink conditions are maximized as the fluid is continually replaced (Brain KR et al., 1998). The donor chamber is, however, very small which gives a small application area, and it is also important to be aware what the system lag-time does to the volume of the receptor chamber and the outlet tubing, which is highly expressed when using a low flow rate. Depending on the amount of cells used in the study, the amount of connecting outlet tubes leading the receptor fluid from the cells to the collecting vials can be quite confusing (see picture below). The mechanical movement, when changing collection vials e.g. every ½ hr., has a tendency to disconnect the tubes.
Figure 9: Flow-through cells (Use of picture permitted by Dr. Wilkinson SC, University of Newcastle).
4.1.3 Advantages/disadvantages
In general the in vitro models have the advantage of avoiding almost all ethical aspect. Since many percutaneous penetration studies would be hazardous to carry out in vivo, e.g. studying chemical warfare agents, the in vitro models meet these risks (Vallet et al., 2007). The static diffusion cells have some advantages compared to the flow-through system, simply due to the much simpler design in the static system. The static diffusion cells have except from the magnetic stirrer no technical features, and this therefore eliminates many of the technical problems that may occur when using the flow-through system. The costs of the static diffusion cells are lower than those of the flow-through system and the static diffusion cells have a larger area of absorption which makes the absorption indicator better as well as the mass balance assessment. The flow-through system, however, provides an environment similar to real physiological conditions by the continuous replacement of receptor fluid (Bronaugh, 2004a;Bronaugh, 2004b) resembling the systemic uptake of the drugs/chemicals in the blood vessels.
Many comparative studies with no difference in skin penetration measurements between the two cell types have been carried out (Bronaugh & Maibach, 1985;Bronaugh & Stewart, 1985a;Chilcott et al., 2005;van de Sandt et al., 2004).
The models are both originally described by OECD guideline 428 (OECD, 2000) for experiments with skin penetration.
4.2 In vivo technique
In vivo technique is based on a physiologically and metabolically intact system. There are two kinds of in vivo studies: 1) animal studies and 2) human studies.
The most frequently used animal is the rat even though it is well known that rat studies generally overestimate human skin absorption (ECETOC, 1993). Other animals demonstrate a better agreement with human absorption, but the costs of these are considerably higher. Human studies are preferable in order to avoid extrapolation between species, but the ethical issues can be extensive.
In 2004 OECD issued a guideline for testing chemicals by in vivo methods. The standard principals described were application of the test substance to the skin in proper form and time, taking samples of different body fluids, excreta or tissue at specific intervals, and quantifying the test substance in the samples or the metabolite in the samples by an appropriately sensitive analytical method.
4.2.1 Traditional in vivo technique
The gold standard is of cause where the test substance is applied to the skin of healthy humans and blood and/or urine is collected and analysed. The amount of test substance measured in the blood and/or urine gives a good indication of the amount of substance absorbed through the skin into the systemic circulation. This in vivo technique has been used before all other techniques were ever considered and is still used where there is no adverse risk to the volunteers participating (Benech-Kieffer et al., 2003;Hueber-Becker et al., 2004;Lammers JHCM et al., 2005;Nohynek et al., 2004;Nohynek et al., 2006;Hueber-Becker et al., 2007). Often the test of a substance is intended to disclose different unknown characteristics of the substance signifying that the knowledge of the substance is limited or incomplete and therefore the risk assessment may not be completely explained. This gives rise to ethical concern and limits the use of healthy volunteers.
4.2.2 Microdialysis
Microdialysis is a technique used in the clinic as well as in research for sampling of endogenous and exogenous substances in the extracellular space in the living tissue. Microdialysis is so far the only technique that provides information from the extracellular space, and it is therefore of great importance in the investigation of pharmacological and biochemical procedures in these tissues. Several results in drug discovery and development are established by measuring serum concentrations of different molecules, even though most drugs exert their effects in the tissues and not in the blood stream. Thus, data on pharmacokinetics at the target site are important, just as determination of pharmacodynamic effects in relation to tissue drug concentrations in the target tissue is a more precise approach to describe exposure effects (Chaurasia et al., 2007). Microdialysis is currently the most essential tool to estimate active drug profiles at the target site and for providing pharmacokinetic and pharmacodynamic information.
Microdialysis is an in vivo sampling technique that can be used to measure endogenous and exogenous substances in the extracellular space in living tissue, e.g. the skin. The method was originally developed in neuropharmacological sciences (Ungerstedt U, 1984), and is now widely used in animal as well as human models to measure substances in different target tissues (Chaurasia, 1999;Kreilgaard, 2002;Lonnroth et al., 1987;Muller, 2002;Stahl et al., 2002), and for investigation of skin absorption. The first report concerning cutaneous microdialysis was published in 1991 (Anderson et al., 1991). This technique involves the insertion of a probe into the dermis (Figure 10).
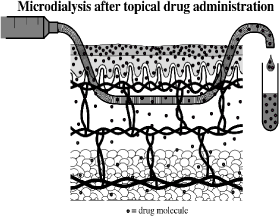
Figure 10: Microdialysis. Probe inserted in the dermis. (Benfeldt & Serup, 1999)
The in vitro techniques have limitations when it comes to studying metabolic, pharmacological and biochemical aspects because of the missing perfusion of the skin. Methods used in vivo – as tape stripping described below – are also inadequate when it comes to looking at metabolism and biochemical characteristics. Here microdialysis has an advantage as the technique makes it possible to examine not only what goes on in the upper layer of the skin but also deeper down in the dermis.
Principals in microdialysis are to imitate the function of a small blood vessel in the dermis. A test substance applied to the skin will penetrate the skin surface to the dermis where the artificial blood vessel/probe is placed. The probes consist of a semi-permeable structure which allows molecules to pass into the perfusate inside the probe by passive diffuse. The molecules follow a concentration gradient across the probe membrane as the perfusate inside the probe passes through, driven at a constant and very accurate pace by a pump. A partial equilibration of molecules across the membrane occurs. The perfusate – now called the dialysate - leaves the probe, holds the test substance and is collected in small vials for analysis. This technique has been used in human volunteers as well as in animals (Benfeldt & Serup, 1999;Benfeldt et al., 2007;Groth, 1996).
4.2.3 Tape stripping
Tape stripping is a well known in vivo technique but can also be used in vitro. A test substance (often radioactively labelled) is allowed to penetrate the skin at a predetermined area for a certain time period. Afterwards the skin is gently washed to remove remaining unabsorbed test substance on top of the skin. The method then involves sequentially removing of microscopic layers of the exposed stratum corneum by repeated application and removal of adhesive tape. The shedded cells attached to the tape are then analysed using an appropriate analytical method.
The method is inexpensive, uncomplicated and a minimally invasive method given that only dead cells (corneocytes) embedded in their lipid matrix are removed.
In dermatopharmacology tape stripping is used to assess cutaneous drugs in the skin after topical dermatological treatment. Tape stripping is a particularly helpful technique to assess the local bioavailability of drugs whose target site is the stratum corneum itself, like e.g. antiseptics (Lboutounne et al., 2002) , antifungal drugs (Alberti et al., 2001b;Alberti et al., 2001a;Pershing et al., 1994) or UVA/UVB filters (Fernandez et al., 2002;Jacobi et al., 2004;Sarveiya et al., 2004;Wissing & Muller, 2002).
The tape stripping method has some disadvantages (see below) but is useful when it comes to studying diseased vs. healthy skin (Jakasa et al., 2004), chemicals that accumulate in the skin, or when it comes to comparing in vitro and in vivo data (Reddy et al., 2002).
4.2.4 Advantages/disadvantages
There are always ethical considerations when choosing an in vivo experimental method. The main advantage is that an in vivo technique uses a physiologically and metabolically active system (OECD, 2004).
The disadvantages when using microdialysis are the cost of pumps, probes and involvement of participants/volunteers. When choosing microdialysis the volunteers have a limited participation time before it becomes uncomfortable to continue staying in the same position. Other considerations are how the test substance interacts with the probe - if it sticks to the probe membrane or not, if the membrane’s permeability - “cut-off” - is suitable for penetration of the specific substance and whether the test substance is suitable for microdialysis or not according to the lipophilicity of the substance – since very lipophilic substances prefer to stay outside the probe. Moreover, a wide range of toxicants will for ethical reasons not be eligible for in vivo human experiments.
Tape stripping is a simple, inexpensive and non-invasive method that can be used in humans as well as in animals. The variation in models used is considerable as the number of tape strips used in removing the stratum corneum varies with the type of tape (Bashir et al., 2001), pressure applied during application and force in removal. Also biological factors give a variation such as anatomical location of application, sex, ethnicity and age of the subject (Palenske & Morhenn, 1999;Loffler et al., 2004). Tape stripping as a stand-alone technique is therefore mainly helpful to assess the local bioavailability of drugs whose target site is the stratum corneum itself.
4.3 In vitro models versus in vivo models
By using an in vitro model the ethical topics are minimized since the use of human volunteers or animals are excluded and the donor skin samples would have been sent to destruction anyway.
The method is excellent looking at the permeability properties of the skin, since they can be maintained when the skin is removed from the body by excision and therefore the stratum corneum, with its principal barrier function, is kept intact.
Other advantages of the in vitro model are the possibilities to replicate the experiment with samples from the same person. Also different species can be studied under identical laboratory conditions and enable comparisons within and between species.
So far it has not been possible to identify any active transport through the skin and since the transportation is believed to be passive diffusion, the barrier that the stratum corneum (non-viable epidermis) consists of is maintained and reliable for in vitro penetration studies.
As mentioned above, the in vitro models are inexpensive to maintain and have limited time consumption.
Results from the in vitro model are easier to reproduce and the method has fewer restricted parameters causing variations. Good association with in vivo experiments has been shown and the method is suitable to predict human transdermal absorption (Scott et al., 1992;van Ravenzwaay B. & Leibold, 2004)}. When choosing an in vitro system with continuous sampling as is seen in the flow-through model, lag-times are often easier to measure, and will therefore not have to be estimated based on a back extrapolation from the linear part of the penetration curve, as is necessary when using the static diffusion cells. Several in vitro models are accepted by the OECD guidelines and qualitative agreement between models is good. Quantitative differences will exist due to e.g. differences in skin thickness between full-thickness skin and dermatomed skin.
In vivo models, however, are the gold standard since they operate using living tissue and operate in a physiologically and metabolically active system. Often the only real limitation is the ethical considerations when using this model.
Version 1.0 May 2009, © Danish Environmental Protection Agency