Dermal absorption of pesticides - evaluation of variability and prevention
9 Concomitant exposure to pesticides and detergents
- 9.1 Approval of commercial products
- 9.2 Penetration profile of commercial products vs. the active ingredient
- 9.3 Mixture of pesticides
- 9.4 Conclusion
9.1 Approval of commercial products
Hazard assessment of pesticides needs to evaluate the toxicity of the active substances as well as the detergent. In this context it is essential also to assess the potential of the detergents to act as mediators of absorption of other potentially toxic substances. Mediators can change the absorption by an increase in the bioavailability of certain products (Sartorelli et al., 1997) or by a direct effect on the skin barrier function (Treffel P & Gabrad B, 1996;TupkerRA, 1990).
Many experiments deal with percutaneous penetration of pesticides, but focus on the active ingredient and do not test the commercial products which are often a mixture of an active substance and a detergent. It is, however, the commercial products that are of interest, since the combination of ingredients may change the penetration characteristics (Baynes & Riviere, 1998;Baynes et al., 2002). Approvals of various compounds and recommendations for protective equipments are often based alone on data from these studies, which as will be shown later can be misleading. Also the US EPA Interagency Testing Committee (ITC) mandated by the Toxic Substances Control Act has required industry to measure in vitro penetration of several chemicals in their pure or neat form (EPA, 2004).
9.2 Penetration profile of commercial products vs. the active ingredient
A comparison study of different commercial products and their active ingredients has been carried out. The penetration rate, lag-time and amount of penetrated substance have been studied and compared by Nielsen (Nielsen JB, 2004).
The commercial product Mesurol® (used to kill snails and slugs in ornamental plants and vegetables) and the active ingredient methiocarb have been studied and the graphs in Figure 15 picturing penetration over time demonstrate significant differences between the product and the active ingredient (Nielsen JB, 2004).
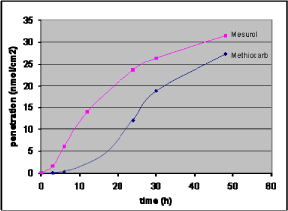
Figure 15: Penetration of Mesurol and the active substance methiocarb. Mesurol as well as methiocarb is applied to the donor chamber in a methiocarb concentration of 0.2 mM. The points represent a mean of 9-11 single data values.
The graph also shows also that the lag-time is reduced from 10 hours to only two hours in the penetration of the commercial product. The decrease in lag-time causes a considerable increased penetration over time and therefore a risk of absorbing larger amounts of chemicals during normal working conditions than assumed based on studies of the active ingredient alone. The knowledge of the difference in lag-time is valuable in relation to advice and guidance related to hygiene and the use of protective gloves when using this pesticide.
The commercial product PirimorG® (used to kill greenfly in agriculture, greenhouses and nurseries) and the active ingredient pirimicarb have been studied and the lag-time of PirimorG® was found to be 2 hours (Table 3), which is significantly lower than the 9 hours observed for the active ingredient (Figure 16).
Table 3: Dermal penetration of PirimorG (pirimicarb) as well as the effect of Mesurol on dermal penetration of PirimorG. The results of penetration and flux are given as mean +/-SEM.
| Pesticid e | N | Penetration of PirimorG after 48 h (nmol/cm²) |
Flux of PirimorG (nmol/cm²/h) |
Lag-time (hrs) |
| PirimorG | 9 | 23.86 ± 2.80* | 0.69 ± 0.11 | 2.1 |
| PirimorG + Mesurol | 12 | 30.64 ± 1.47* | 0.95 ± 0.08 | 4.7 |
* - significant differences; Students t-test, p<0.01.
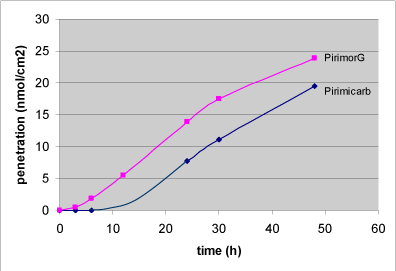
Figure 16: Penetration of PirimorG and the active substance pirimicarb. PirimorG as well as pirimicarb were applied in the donor chamber in a pirimicarb concentration of 0.2 mM. The points represent a mean of 9-11 single data values.
The penetration rate, however, is similar in both substances, thus only 25% more active substance penetrates the skin in 48 hrs when testing the commercial product compared to the pure active substance. But as mentioned before the reduced lag-time is highly relevant when looking at risk assessment and working conditions.
The condition of the substance may also be important when evaluating permeation of chemicals. Most laboratory studies measure chemical penetration from an aqueous solution through isolated human or animal skin, although most exposures are not from pure aqueous solutions. Frasch et al. have measured skin permeability and lag time for three neat chemicals of industrial importance (diethyl phthalate (DEP, slightly volatile), 1,2-dichloroethane (DCE, highly volatile), and naphthalene (NAP, solid)) and found the maximum measured flux of saturated aqueous DEP was ~ 2 times greater than that from neat DEP but lower with DCE. Maximum flux of neat DCE was nearly 6 times higher than DCE in a saturated aqueous buffer. The maximum penetration rate of solid NAP powder was ~ 2 times higher than the rate from a saturated aqueous solution (Frasch et al., 2007). NAP is known to be a strong skin irritant (IPCS INCHEM, 2002), which may be the reason for the increased permeation rate. Studies on other chemicals have, however, not been able to demonstrate particular penetration differences between saturated aqueous solutions and powder (McCarley & Bunge, 2003;Romonchuk & Bunge, 2006).
9.3 Mixture of pesticides
Commercial formulations contain detergents and other compounds to increase absorption by targeted plants or to ease mixing and distribution in aqueous solutions. These chemicals, however, are also potential penetration enhancers for human skin. A mixture of PirimorG® and Mesurol® give us an indication of how two substances may influence each other. The results are shown in Tables 4 and 5 and the interaction between the two products is absolutely diverse.
Table 4: Dermal penetration of Mesurol measured as methiocarb, as well as the effect of PirimorG on dermal penetration of Mesurol. Results on penetration and flux are given as mean +/-SEM.
| Pesticide | N | Penetration of Mesurol after 48 h (nmol/cm²) |
Flux of Mesurol (nmol/cm²/h) |
Lag-time (hrs) |
| Mesurol | 9 | 31.44 ± 2.46 | 1.39 ± 0.22 | 1.9 |
| Mesurol + PirimorG | 12 | 32.51 ± 1.16 | 1.25 ± 0.15 | 3.4 |
Table 5: Effect of combined exposure of methiocarb (0.2mM) or pirimicarb (0.2mM), as well as the effect of three detergents in relation to the dermal penetration and flux of paclobutrazol (0.2mM). Results are given as mean +/-SEM.
| Pesticide exposure |
Detergent | N | Penetration of methiocarb after 48 hrs (nmol/cm²) |
Flux of methiocarb (nmol/cm²/hr) |
Lag-time (hrs) |
| Paclobutrazol (paclo) | - | 17 | 12.09 ± 1.57* | 0.37 ± 0.04* | 15.3 |
| Paclo+ methiocarb | - | 11 | 9.28 ± 0.96 | 0.38 ± 0.06 | 17.0 |
| Paclo+ pirimicarb | - | 11 | 21.51 ± 1.47* | 0.73 ± 0.09* | 13.1 |
| Paclo+ methiocarb | PG | 10 | 11.08 ± 1.23 | 0.35 ± 0.03 | 10.1 |
| Paclo+ methiocarb | SLS | 9 | 10.12 ± 0.94 | 0.34 ± 0.05 | 12.9 |
| Paclo+ methiocarb | EG | 9 | 9.39 ± 0.83 | 0.28 ± 0.05 | 11.8 |
| Paclo+ pirimicarb | PG | 9 | 18.13 ± 1.65 | 0.65 ± 0.08 | 13.5 |
| Paclo+ pirimicarb | SLS | 10 | 18.55 ± 1.77 | 0.56 ± 0.07 | 10.9 |
| Paclo+ pirimicarb | EG | 10 | 19.78 ± 2.73 | 0.58 ± 0.10 | 11.7 |
PG – Propylene glycol (10mM); SLS – Sodium Lauryl Sulphate(7mM); EG – Ethylene glycol (10mM). * - significant differences; Students t-test, p<0.001.
PirimorG® did not affect the penetration of methiocarb from Mesurol® whereas the opposite is the case when it comes to penetration of PirimorG®. There is a clear change in penetration characteristics showing a 40% increased flux of pirimicarb in combination with Mesurol®. This gives a 30% increased penetration after 48 hours. The reason for this increase in flux should probably be found in the effect of the detergents present in the commercial products since it cannot be detected in the studies of the pure active substances. There is a good correlation between these findings and the changes that was found in the studies of the pure active substance compared to the commercial products where a clear increase in penetration was also found (Nielsen JB, 2004).
The data demonstrate that some substances can increase the permeability of others, as well as decrease the lag-time. The increase in penetration of active substances when testing commercial products is in good correlation with other studies of chlorpyrifos, atrazine, arachlor and trifluralin, where a significant increase in dermal penetration of the four pesticides are seen when they are administered as commercial products (Brand & Mueller, 2002;Griffin et al., 2000)
The evidently shorter lag-time seen in the studies of Mesurol® and PirimorG® signifies that under normal working conditions the absorption of those two substances happens faster than originally assumed given that earlier studies are made on active substances alone and not the commercial products.
But also other kinds of compounds applied to the skin before handling pesticides can change the permeation as seen in a recent study made by Brand et al.(Brand et al., 2007). They tested four commercially available moisturizing creams and their capacity as transdermal penetration enhancers using the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) as a model compound. Their data demonstrated that pre-treatment with three of the four creams increased the absorption of 2,4-D as evidenced by either an increased cumulative penetration or shorter lag-times. Also skin barrier creams have demonstrated to enhance the penetration rates of different industrial solvents in a study by Korinth 2003 and the creams significantly enhanced the penetration rates of solvents from complex mixtures compared with the single solvents. (Korinth et al., 2003a).
9.4 Conclusion
It is known that the nature of the vehicle in which a substance is applied to the skin may change the absorption rate, lag-time or both by either increasing it or slowing it down. The reduced lag-time demonstrated the range of effects that different detergents may have on penetration of pesticides, and suggests that regulatory agencies consider these data when evaluating pesticides resubmitted for approval after a change in formulation. In most cases the Danish EPA does not require updated pesticide-specific penetration data when re-evaluating known pesticides in changed formulations, but rely on extrapolation from other products as well as physic-chemical comparisons between detergents. This procedure is complicated and requires considerable toxicokinetic as well as chemical insight. The EPA should consider how large differences can be before submission of supporting data on penetration should be required.
To the people handling these products, e.g. greenhouse workers, farmers, house and garden owners, this information is also important knowledge and advice and guidance must be carefully considered so that these people take the necessary precautions, e.g. hygiene and the use of gloves as protective equipment.
Version 1.0 May 2009, © Danish Environmental Protection Agency