Dermal absorption of pesticides - evaluation of variability and prevention
10 Penetration of pesticides through slightly damaged skin
- 10.1 OECD guidelines
- 10.2 Work-related skin problems
- 10.3 Trans Epidermal water Loss (TEWL)
- 10.4 Skin-damaging factors
- 10.5 Affecting skin integrity
- 10.6 Conclusion
The more we know about the ideal and optimal situation, the more important our knowledge about the exceptions get. Because many people have a decreased integrity of their skin barrier, it is important to adjust the experimental studies to real-life situations and use experimental models where it is possible to study percutaneous penetration through slightly damaged skin (Kezic & Nielsen JB, 2008). A minor damage to the skin is expected to increase the penetration, but whether this is caused by a decreased lag-time or an increased penetration rate (flux) needs further investigation. It is also relevant to study whether an effect on penetration might be observed to the same extent in more or less hydrophilic or lipophilic pesticides, though recent data on a number of test substances indicate the effect of lipophilicity on permeability characteristics (Borras-Blasco et al., 2004). In other words, the presence of a mixture of detergents/vehicles, as in the formulated pesticide products (sales formulations), may influence penetration characteristics as indicated through accessible data on the effect of detergents on the integrity of the skin barrier (Dupuis et al., 1986;Nielsen et al., 2000;Nielsen, 2000).
10.1 OECD guidelines
The most reliable information on dermal absorption for the use of human health risk assessment is achieved from occupational field studies and controlled exposure studies in volunteers. However, due to technical and ethical restrictions these studies are limited.
Therefore, in experimental percutaneous penetration studies it has over the years been common to use a number of different techniques and methodologies based on skin samples from humans (cadavers or surgical waste) or animals (rats or pigs). Recently guidelines have been drawn towards more standardised methods, and the latest guideline from Organisation for Economic Cooperation and Development (OECD) is one of the outcomes (OECD, 2004). The main purpose of guidelines are to assure an acceptable quality in the output of an experiment and by that the possibility to compare results from different laboratories. A general characteristic of guidelines on experimental studies of percutaneous penetration, including the latest from OECD, is the attempt to aensure intact, homogenous, and optimal barrier conditions of the skin samples used. Thus, specific requirements are listed to ensure the integrity of the skin barrier, which must be ensured and documented through measurement of trans-epidermal water loss (TEWL), electrical resistance or capacitance (OECD, 2000;Davies et al., 2004). Thus, the OECD guidelines used in hazard and risk evaluations prescribe experimental conditions with optimal barrier integrity of the skin, which in many occupational settings probably is not true.
10.2 Work-related skin problems
The skin exposure in daily work – household or occupational situations - is not always ideal. Skin problems are among the most dominant reasons for absence from work in many countries. Occupational contact dermatitis is the most frequent occupational skin disease with an estimated average incidence rate of 0.7-1.5 cases per 1,000 workers per year according to a German study (Elsner, 2007). In another German study the prevalence of damaged skin was close to 70% in the printing industry and approximately 80% among workers in the rubber industry (Korinth et al., 2005;Korinth et al., 2007b;Korinth et al., 2007c). Yet another study from Finland finds an incidence of occupational diseases of 5.9 cases per 1000 person-years among machinists and 2.7 cases per 1000 person-years in the total workforce. Of these occupational diseases in machinists 27% were skin-related problems (Suuronen et al., 2007).
Epidemiological studies indicate that only 4-9% of people with work-related skin problems are on the sick list and the true prevalence of skin disorders within the active work force is believed to be a lot higher than official statistics (Diepgen, 2003;Hadgraft, 2004). A Danish study describes an incidence of work-related dermatological diseases in 17,700 people out of a total work force of 2.6 mio. (Halkier-Sorensen, 1996), thus, 6.8 cases per 1000 persons. The number of self-reported cases of hand eczema in a hospital population showed an overall frequency of hand eczema in 12 months of 22.8% in an unselected population of hospital employees in a recent Danish study (Flyvholm et al., 2007).
Data from the U.S. point out that one fourth of nurses met the criteria for currently damaged skin on the hands (Larson et al., 1997), and the damage has proven to be related to use of gloves and handwashing practices.
A Swedish study showed that occupational dermatological problems often end up as chronic conditions with a majority reporting symptoms at a 12-year follow-up. A skin disease influences the occupational situation for the majority (82%) and for 15% results in exclusion from the labour market through unemployment or disability pension (Meding et al., 2005).
These few examples illustrate the magnitude of the problem in high risk occupations with skin exposure to solvents, water and detergents.
Affected skin barrier due to continuing wet work, small bruises, skin irritation, eczema etc. has proven to increase dermal absorption (Bronaugh & Stewart, 1985b;Grandjean P, 1990;Ilyin et al., 1975;Scott & Dugard, 1986). Accordingly, industrial hygienists have for several years anticipated, and advised workers, that a decreased integrity of the skin barrier may increase percutaneous penetration of chemicals (Grandjean P, 1990;Levang et al., 1999).
10.3 Trans Epidermal water Loss (TEWL)
Damage of the skin barrier implies an increase in TEWL. TEWL is the normal, constitutive loss of water vapour from the skin in absence of sweat gland activity (Gioia & Celleno, 2002). In a number of studies good correlation between TEWL and drug penetration of different substances has been demonstrated (Benfeldt et al., 1999;Tsai et al., 2001). In a recent study on the validity of TEWL, comparisons to a variety of methods in a variety of models demonstrated that TEWL was a reliable tool for the assessment of variations in permeability and barrier function (Fluhr et al., 2006).
However, Chilcott et al. reported that TEWL did not reflect permeability of sulphur mustard - a lipophilic substance (Chilcott et al., 2002) and Tsai et al. showed absence of correlation between TEWL and dermal absorption of highly lipophilic substances (estradiol and progesterone) in acetone pre-treated skin (Tsai 2001). Benfeldt et al. found a 2.2-fold increase in dermal absorption of salicylic acid through acetone-treated skin although TEWL was not different from untreated skin (Benfeldt et al., 1999). Regardless of a poor correlation between TEWL and dermal absorption for some lipophilic substances described in different studies it is suggested that TEWL may be used as a predictor of barrier integrity and dermal absorption of hydrophilic and slightly lipophilic substances (Levin & Maibach, 2005).
TEWL has shown to be useful in studies evaluating different skin types/thicknesses or barrier qualities. A recent study compared heat-separated epidermis and dermatomed skin in a qualitative and quantitative percutaneous absorption study and used TEWL as endogenous standard (Atrux-Tallau et al., 2007).
10.4 Skin-damaging factors
10.4.1 Detergents
Detergents are also known as surfactants and are described in section 8.1. They can enhance skin permeability by a range of mechanisms, together with enhanced solubility and increasing partitioning into the stratum corneum. Certain anionic surfactants such as sodium lauryl sulphate (SLS) affect not only the barrier itself but during prolonged exposures also the underlying viable cell layers leading to skin inflammation and possibly further deterioration of the skin barrier. The effect of SLS on the skin penetration has been shown to depend on the lipophilicity of the penetrant (see below in section 10.4.1)
10.4.2 Organic solvents
An organic solvent is a (carbon-containing) chemical that dissolves a solid, liquid, or gaseous solute, resulting in a solution. They are widely used as degreasers, cleaning agents, solvents for plastics, paints and rubber in numerous industrial applications. Data from England estimate that two million workers have regular contact with solvents (Semple 2004). Solvents are responsible for as much as 20% of all cases of occupational dermatitis because of their severe effect on the skin (Kamijima et al., 2007). The effects are local irritant reactions, epidermal necrosis and cytotoxicity associated with oxidative stress (Rowse & Emmett, 2004).
Often exposures to solvents occur when skin contacts a liquid, but also exposures to vaporous mixtures have shown to damage the skin barrier (Huss-Marp 2006).
10.4.3 Hydration of the skin
To keep a good skin barrier and an optimized barrier function it is important that the skin is sufficiently hydrated. When the skin becomes dry the flexibility and elasticity of the skin decreases. This can lead to cracks in the stratum corneum and a vulnerable skin barrier. In some occupations, such as in lithium battery manufacturing and the pharmaceutical industry a low room humidity under 40-50% is required. Working in this environment for a long time may cause dry and xerotic skin (Sato et al., 2003).
Also increased hydration may alter the skin permeability (Warner et al., 2003). This may occur following working in a wet environment, which is the case for health care personnel, housekeepers, hairdressers and dishwashers who are in frequent contact with water. But also conditions where normal water evaporation is prevented by wearing of gloves and protective clothing will increase skin humidity. These occupations are known to have a high prevalence of contact dermatitis.
Depending on the air humidity, the water content of stratum corneum is around 40% of the tissue dry weight (Williams & Barry, 2004). When occluded the stratum corneum will absorb a lot more and this can affect the permeability of the skin to chemicals (Rawlings & Harding, 2004;Rawson et al., 2005;Scheuplein & Blank, 1971;Warner et al., 2003;Wester & Maibach, 1983). Idson (1971) claimed that increasing skin hydration enhances the absorption of all substances that go through the skin (Idson, 1971). However, increased hydration (due to occlusion) does not always increase penetration rates. Bucks et al. (1991) stated that hydration reduced the penetration rate of hydrophilic compounds like hydrocortisone (Bucks et al., 1991). In contrast, Wurster & Kramer (1961) observed that occlusive covering that prevented water loss at the same time increased the dermal absorption of some hydrophilic compounds. Increased skin hydration has been mentioned as the likely cause of the increase in absorption, although in most studies contributions from potentially misleading things such as increased temperature or accumulation of sweat cannot be rejected (Fluhr et al., 1999;Wurster & Kramer, 1961;Zhai & Maibach, 2001).
Jones et al. showed that dermal absorption of 2-butoxyethanol in humans was enhanced significantly when air humidity increased from 40% to 65% (Jones et al., 2003). Dermal absorption of propoxur at different levels of humidity between 50 and 90% showed a linear relationship between the level of air humidity and the level of skin humidity (Meuling et al., 1997). Also different parabens (methyl-paraben and butyl-paraben) have an increased absorption during occlusion (Akomeah et al., 2004).
10.4.4 Mechanical damage
Damage to the skin can also be done mechanically by abrasion, scrubbing and skin friction and will result in disruption of the skin barrier. To imitate mechanical skin damage the stratum corneum may be removed by tape-stripping (see chapter on Methods to study dermal penetration). Morgan et al. found in a microdialysis study a correlation between the extent of damage to the stratum corneum and the penetration of hydrophilic aciclovir and penciclovir. A total removal of the stratum corneum increased the penetration by 400-1300-fold in contrast to normal skin (Morgan et al., 2003). In another microdialysis study Benfeldt et al investigated the penetration of the hydrophilic salicylic acid and showed an increased absorption of 150-fold in stripped skin (Benfeldt et al., 1999). Akomeah et al. studied the effect of abrasion induced by a rotating brush on the skin permeation of solutes with varying physiochemical properties and found a significant increase in permeability on especially the hydrophilic substances (Akomeah et al., 2008).
Furthermore, penetration of lipophilic substances is also affected by a damaged skin barrier. Less than 1% penetration was observed with intact skin, whereas up to 23% of latex proteins applied to abraded skin was recovered in the receptor fluid within 24 h of exposure (Hayes et al., 2000).
10.4.5 Pathologically compromised skin barrier
Compromised skin barrier can be an inherent skin barrier defect. Suffering from atopic dermatitis and lamellar ichthyosis will change the skin profile with altered fatty acids and ceramide content (Imokawa et al., 1991;Imokawa, 2001;Yamamoto et al., 1991). Also a mutation in filaggrin which is a key protein of the stratum corneum may alter the skin barrier (Palmer et al., 2006;Chen et al., 2008). (see section 5.3).
The majority of children suffering from atopic dermatitis demonstrate filaggrin mutations (Palmer et al., 2006). An increase in the occurrence of this phenomenon over the last decade of 15-20% is a huge concern (Vasilopoulos et al., 2004). As a result of alterations in barrier function, it is possible that larger substances such as allergens and irritants may enter the skin (Proksch et al., 2003).
Patients suffering from atopic dermatitis have an altered skin barrier not only in the inflammatory area but also in the skin with a clinically normal appearance (Seidenari & Giusti, 1995). Furthermore, de Jongh revealed a higher permeability in individuals with atopic constitution but without manifestation of atopic dermatitis (de Jongh et al., 2006).
Also Jakasa et al. showed a higher diffusion of SLS and polyethylene glycol of a different molecular size through not affected skin in patients suffering from atopic dermatitis and demonstrated a relative increase in penetration after damage to the skin barrier in the glycol with the highest molecular size (Jakasa et al., 2006b;Jakasa et al., 2007). A study of offset printing workers exposed to a glycol ether also demonstrated that erythema and scaliness were important parameters affecting skin absorption (Korinth et al., 2003b). Patients suffering from impaired genetic inclination to produce increased levels of stratum corneum or suffering from an imperfection in enzymes causing premature breakdown of corneodesmosome will experience damage of the stratum corneum (Cork et al., 2006).
10.4.6 Distribution within the skin
Test substances are expected to move between compartments based on plain diffusion along a concentration gradient, though movement from one compartment to another will also depend on the lipophilic character of the two compartments. If the skin compartment is more lipophilic than the solution in which the substance is dissolved, lipophilic substances are expected to prefer to stay in the skin compartment, whereas more hydrophilic substances may prefer to stay in the solution. If the concentration gradient becomes sufficiently large, the hydrophilic substance might get forced into the skin membrane, but then instantly continue to the more hydrophilic receptor chamber. In contrast, lipophilic substances will be expected to stay in the skin compartment until the concentration gradient is large enough for the substance to pass through. In a medical situation we might want to create a substance that will penetrate the skin into the general circulation. To do this the substance needs to be lipophilic enough to want to pass into the skin membrane and also be hydrophilic enough to want to leave the skin and enter into the bloodstream.
These aspects may partly explain the prolonged lag-time of hydrophilic substances as well as the absence of any significant penetration of lipophilic substances. It is therefore important in experimental studies not to underestimate the consequence of the lipophilicity in the receptor phase.
Lag-time depends on the distance that a substance needs to move to get from the donor chamber to the receptor chamber. The distance is due to the lamellar structure of the stratum corneum considerably longer than the straight measurable thickness of the stratum corneum (Borras-Blasco et al., 2004). By changing the lamellar structure or the physiochemical characteristics of the stratum corneum it is therefore possible to manipulate the lag-time and the flux. Flux at steady state will, however, as previously mentioned also depend on the rate constant for transport from donor into the skin compartment and from the skin out into the receptor phase.
Changing the structure may reduce the distance across the skin membrane but may not necessarily affect the rate of transfer. Dermal penetration modifiers may in other words affect lag-time and flux separately as well as together (Figure 17).
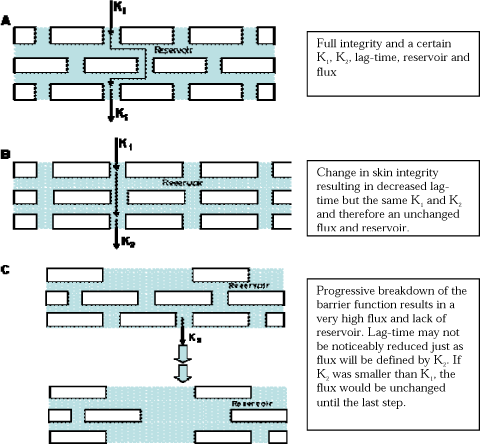
Figure 17. Changes in skin barrier leads to changes in permeability as visualised in Figures A, B and C.
Commercial products usually contain a number of detergents that act as modifiers of solubility and/or penetration characteristics of the active ingredient. Experiments have shown that detergents presented in commercial products can act by enhancing the maximal flux without having any affect on the lag-time or decrease the lag-time without affecting the flux (Nielsen, 2005a).
Sodium lauryl sulphate (SLS) is another modifier that acts through changes in the skin integrity. The effect of pre-treatment with SLS is generally an increased maximal flux and a reduced lag-time. The increased flux is, however, only significant for the most hydrophilic test substances (Borras-Blasco et al., 2004;Nielsen, 2005a).
10.5 Affecting skin integrity
Inducing a universal impairment of the skin integrity should on one hand encourage a measurable, stable and permanent damage, but on the other hand not compromise the integrity to a degree that will take away all barrier properties.
Compromised integrity of the skin may be achieved chemically as well as mechanically through continual tape-stripping or abrasions by use of a scalpel blade or sand paper (Ilyin et al., 1975;Scott et al., 1986;Proksch et al., 1996).
A well-known treatment of the skin in order to produce a damage of the barrier is to treat the skin for a specific time period with sodium lauryl sulphate (SLS). This method is more reproducible than consecutive tape-stripping. SLS is often used as a positive control in studies on irritation or effects of chemical exposure on the barrier integrity of the skin (Dickel et al., 2004;Nielsen, 2000) as well as used as a penetration enhancer in drug formulations (Piret et al., 2000;Nokhodchi et al., 2003;Borras-Blasco et al., 2004). Damage of the skin barrier integrity by SLS has previously been attributed to removal of intercellular hydrophilic lipids, which may be observed through an increase in TEWL. (Froebe CL 1990). A later study has indicated that SLS fluidizes the lipid bilayers in the stratum corneum (Ribaud et al., 1994). These two effects are suggested to increase percutaneous penetration of primarily hydrophilic compounds (Akomeah et al., 2007;Akomeah et al., 2008), whereas the percutaneous penetration of highly lipophilic compounds should remain unaffected (Borras-Blasco et al., 2004). It has been demonstrated that concentrations below 1% SLS are sufficient to produce an impaired barrier function (Figure 19) and that an increase in SLS concentration above 1% will not further enhance percutaneous penetration of lipophilic test substances (Borras-Blasco et al., 2004;Nielsen, 2000). By using SLS it is easy to establish reproducible skin damage and the use of different SLS concentrations has the advantage of inducing different degrees of damage.
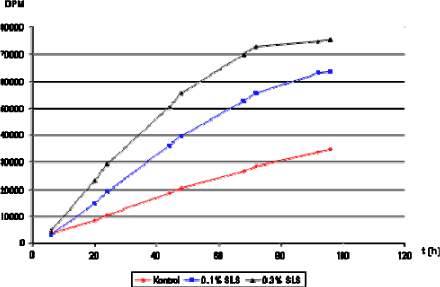
Figure 18. Penetration of tritiated water after pre-treatment with SLS for three hours in different concentrations (0.1% and 0.3%). The concentration of tritiated water was identical in all three groups and is expressed as disintegrations per minute (DPM).
Studies have shown an intraexperimental coefficient of variation between 11% and 16% which is an acceptable reproducibility, especially when the natural heterogeneity among human skin samples is considered (Nielsen, 2005).
Lag-time and flux determine the amount of chemical that may be observed penetrating the skin within a certain time period. Both parameters have previously been demonstrated to depend on the lipophilicity of the test substances used in studies on percutaneous penetration of skin with full integrity (Nielsen et al., 2004). Nielsen et al. 2006 demonstrated an increase in maximal penetration rate leading to an increased penetration coefficient (Kρ) in 6 test substances. The increase in Kρ of benzoic acid is not significant, which is probably because benzoic acid already penetrates undamaged skin easily, has a low molecular size and therefore has no obvious gain of the skin barrier being compromised.
More lipophilic substances – like malathion and methiocarb - show an increase in Kρ of 90% and 15% respectively (Nielsen JB et al., 2006)
The difference in penetration rate through undamaged versus damaged skin may be due to the fact that malathion has a larger molecule size and therefore acquires greater advantage when the skin barrier is damaged than a chemical with a small molecule size will (illustrated in Figure 19 and Table 6 (Nielsen et al., 2007).
Table 6: Penetration characteristics of six test substances from Nielsen et al. 2006. The results are stated as mean values of data from 11-15 penetration cells.
| Intact Non-washed |
Damaged Non-washed |
Intact Washed |
Damaged Washed |
Sales product Intact Non-washed |
Sales product Damaged Non-washed |
|
| Kρ (um/h) | ||||||
| Glyphosate | 0,04 | 0,97 | 0,04 | 0,07 | ||
| Caffeine | 4,5 | 21,7 | 2,5 | 14,3 | ||
| Dimethoate | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzoic acid | 51,1 | 53,8 | 39,5 | 60,7 | ||
| Malathion | 4,5 | 8,4 | 4,6 | 11,6 | ||
| Methiocarb | 39,5 | 45,5 | 30,0 | 35,0 | 47,0 | 35,5 |
| Lag-time (h) | ||||||
| Glyphosate | 3,3 | 8,7 | 4,2 | 4,1 | ||
| Caffeine | 6,5 | 5,9 | 5,0 | 4,8 | ||
| Dimethoate | - | - | - | - | - | - |
| Benzoic acid | 1,8 | 1,7 | 1,7 | 1,5 | ||
| Malathion | 2,6 | 2,2 | 3,0 | 2,6 | ||
| Methiocarb | 15,7 | 11,7 | 14,3 | 8,5 | 13,8 | 10,9 |
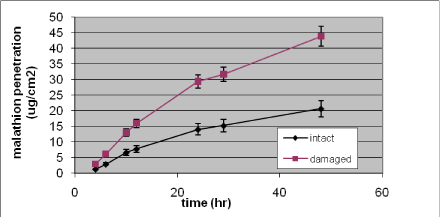
Figure 19. In vitro percutaneous penetration of malathion through intact and slightly damaged human skin. A total amount of 424 µg (2 mg/ml) was added to the donor chamber and penetration followed for 48 h. Results are presented as mean ± SEM (n = 13–14 per group)
A clearer increase in penetration rate is seen in the hydrophilic substances. An example is the graphs below where a 500% increase in penetration rate of caffeine is seen and an increase of 25-fold in penetration rate of glyphosate is also demonstrated (Figures 20 and 21) (Nielsen et al., 2007). Jakasa et al. came to the same conclusion when studying the permeability of PEG in compromised skin (Jakasa et al., 2006b).
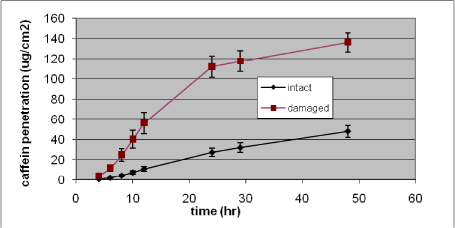
Figure 20. In vitro percutaneous penetration of caffeine through intact and slightly damaged human skin. A total amount of 424 µg (4 mg/ml) was added to the donor chamber and penetration followed for 48 h. Results are presented as mean ± SEM (n = 14 per group)
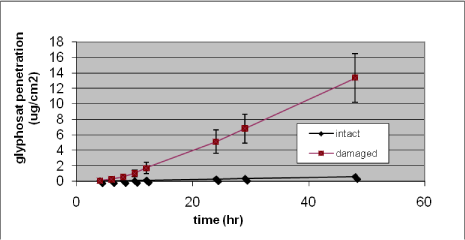
Figure 21. In vitro percutaneous penetration of glyphosate through intact and slightly damaged human skin. A total amount of 424 µg (4 mg/ml) was added to the donor chamber and penetration followed for 48 h. Results are presented as mean ± SEM (n = 13–14 per group)
10.6 Conclusion
Damage of the skin undoubtedly has an effect on the permeability coefficient (Kρ), penetration rate, lag-time as well as the total penetration of chemicals with different solubilities. An increase in permeability coefficient Kρ is most significant for those substances that due to their physicochemical characteristics (the most hydrophilic as well as the most lipophilic) have low penetration rates through intact skin. Substances having a hard time penetrating intact skin will in slightly damaged skin achieve a greater gain in their penetration characteristics. The hydrophilic compounds will be those most affected by a change in skin barrier integrity since it is the step from the donor chamber into the more lipophilic skin that is affected. This part of the absorption is uncomplicated to lipophilic substances.
This significant increase in penetration of especially the hydrophilic substances may well have the consequence that exposed employees, suffering from damaged skin, will absorb considerably more through the skin than traditional in vitro experiments have predicted.
The knowledge of impaired skin integrity of many exposed employees and also of people in households should clearly be considered when setting standards for dermal exposure to chemicals. Furthermore, SLS is extensively used as a detergent in many products including household products and soaps. Even in small concentrations the effect of SLS is obvious and this knowledge should motivate producers of these products to use other detergents that to a much lesser degree affect the skin barrier.
Version 1.0 May 2009, © Danish Environmental Protection Agency