Toxicological evaluation and limit values for Methyl-tertiary-butyl ether (MTBE), Formaldehyde, Glutaraldehyde, Furfural
Table of contents
Preface
Principles for setting of limit values for chemical substances
Methyl-tertiary-butyl ether (MTBE)
1. General description
1.1 Identity
1.2 Physical/chemical properties
1.3 Production and use
1.4 Environmental occurrence
1.5 Environmental fate
1.6 Human exposure
2. Toxicokinetics
2.1 Absorption, distribution
2.2 Elimination
4. Toxicity, animal data
4.1 Short term toxicity
4.2 Long term toxicity
4.3 Reproductive and developmental effects
4.4 Mutagenic and genotoxic effects
4.5 Carcinogenic effects
8. TDI, health based limit values
8.1 TDI
8.2 Limit value in soil
8.3 Limit value in drinking water
8.4 Limit value in air
9. C-value
9.1 Quality criteria in soil
9.2 Quality criteria in drinking water
9.3 C-value
Formaldehyde
1. General description
1.1 Identity
1.2 Physical/chemical properties
1.3 Production and use
1.4 Environmental occurrence
1.5 Environmental fate
1.6 Human exposure
2. Toxicokinetics
2.1 Absorption, distribution
2.2 Elimination
3. Human toxicity
3.1 Short term toxicity
3.2 Long term toxicity
3.3 Reproductive / developmental effects
3.4 Genotoxic effects
3.5 Carcinogenic effects
4. Toxicity, animal data
4.1 Short term toxicity
4.2 Long term toxicity
4.3 Reproductive / developmental effects
4.4 Genotoxic effects
4.5 Carcinogenic effects
4.6 Combination effects
8. TDI, health based limit values
8.1 TDI
8.2 Limit value in soil
8.3 Limit value in drinking water
8.4 Limit value in air
9. C-value
9.1 Quality criteria in soil
9.2 Quality criteria in drinking water
9.3 C-value
Glutaraldehyde
1. General description
1.1 Identity
1.2 Physical/chemical properties
1.3 Production and use
1.4 Environmental occurrence
1.5 Environmental fate
1.6 Human exposure
2. Toxicokinetics
2.1 Absorption, distribution
2.2 Elimination
2.3 Toxicological mechanisms
3. Human toxicity
3.1 Short term toxicity
3.2 Long term toxicity
3.3 Reproductive / developmental effects
3.4 Genotoxic effects
3.5 Carcinogenic effects
4. Toxicity, animal data
4.1 Short term toxicity
4.2 Long term toxicity
4.3 Reproductive / developmental effects
4.4 Genotoxic effects
4.5 Carcinogenic effects
Furfural
1. General description
1.1 Identity
1.2 Physical/chemical properties
1.3 Production and use
1.4 Environmental occurrence
1.5 Environmental fate
1.6 Human exposure
2. Toxicokinetics
2.1 Absorption, distribution
2.2 Elimination
2.3 Toxicological mechanisms
3. Human toxicity
3.1 Short term toxicity
3.2 Long term toxicity
3.3 Reproductive and developmental effects
3.4 Mutagenic and genotoxic effects
3.5 Carcinogenic effects
4. Toxicity, animal data
4.1 Short term toxicity
4.2 Long term toxicity
4.3 Reproductive and developmental effects
4.5 Carcinogenic effects
8. TDI, health based limit values
8.1 TDI
8.2 Limit value in soil
8.3 Limit value in drinking water
8.4 Limit value in air
9. C-value
9.1 Quality criteria in soil
9.2 Quality criteria in drinking water
9.3 C-value
Preface
This series of reports constitutes a part of the work related to the setting of health based limit values for chemical substances in air, soil and drinking water.
In this report, the toxicological documentation for the setting of limit values for nonylphenol and nonylphenol ethoxylates, tricresylphosphates and benzoic acid are presented. For every substance, the following items are considered:,
part 1, physicochemical properties, production and uses, environ mental occurrence and
fate, and human exposure
part 2, toxicokinetic properties and toxicological mechanisms
part 3, human toxicity
part 4, animal toxicity
part 5, regulations and limit values in different media
part 6, summary of part 1 to 5
part 7, evaluation of toxicity and identification of critical effects
part 8, estimation of tolerable daily intake (TDI) and health based
limit values
part 9, implementation of limit values to quality criteria
The work has been carried out by the Institute of Food Safety and Toxicology, Danish Veterinary and Food Administration as a contract work for the Danish Environmental Protection Agency. The work has been followed by a Steering Committee who has contributed to the work with professional expertise, proposals and criticism:
Linda Bagge, Chairman, Danish Environmental Protection Agency Poul Bo Larsen, Danish Environmental Protection Agency Erik Thomsen, Danish Environmental Protection Agency Hans Chr. Ellehauge, Danish Environmental Protection Agency Anders Carlsen, Medical Health Office for Viborg County Elle Laursen, National Board of Health Ole Ladefoged, Institute of Food Safety and Toxicology Elsa Nielsen, Institute of Food Safety and Toxicology
Principles for setting of health based limit values for chemical substances
In the following, the principles upon which the Danish Environmental Protection Agency bases the health based limit values, in the following referred to as limit values, for chemical substances are briefly outlined. For further and more specific information, the reader is referred to the references mentioned below.
Purpose
The purpose of setting limit values for chemical substances is to prevent health hazards in the human population caused by chemicals as pollut ants. The scientific method for setting of limit values comprises a hazard identification and hazard assessment which together with an exposure assessment constitute the risk assessment part in the proces of setting limit values.
Selection of data
Data concerning exposure and harmful effects of a chemical substance are collected from national and international criteria documents, mono graphs and original scientific literature. During the review of the data, the quality and reliability of the studies and research work are critically assessed. This is an important step since conflicting viewpoints regarding the hazards may be present. Unpublished data from industry or other sources are only seldom used, as such data have not been published in scientific journals and have not been subjected to critical review by other scientists.
If adequate human data are available these are preferred as the basis for the assessment. For most substances however, human data are not ade quate or available. In these cases, limit values are based upon data from experimental animal studies.
When all the relevant data have been evaluated, the hazard considered most important - "the critical effect" - for setting the limit value, is iden tified. In this step it is assessed whether an effect should be considered as adverse and of relevance to humans.
A substance may have different effects at different concentrations or doses. Generally, the effects are of more concern the lower the concen tration or dose at which they occur, and the effect observed at the lowest concentration or dose often forms the basis for setting the limit value.
Threshold chemicals,
The next step for assessment of a limit value is to identify the "no ob NOAEL or LOAEL served adverse effect level" (NOAEL) which is the highest dose at which the critical effect was not observed or, in cases where a NOAEL cannot be identified, the "lowest observed adverse effect level" (LOAEL) which is the lowest dose at which the critical effect was observed.TDI /safety factors
Having identified a NOAEL or a LOAEL, three "safety factors" (SF) are used to extrapolate from NOAEL or LOAEL to the tolerable daily intake,TDI (expressed in mg/kg b.w. per day) or the limit value for air, LVa;r,
![]()
![]()
(expressed in mg/m3). The purpose of the safety factors is to take into account the fact that:
SF,: The toxicological effect of a chemical substance on animals need not reflect the toxicological effect on "normal" humans, this factor is historically set at 10.
SF2: The toxicological effect of a chemical substance may vary considerably between different persons, and that i.e. children, elderly or sick people may be much more sensitive to exposure than "normal" people, this factor is often set at 10.
SF3: The data may be of varying quality and relevance to the actual problem, this factor is set at a value from 1 to 1000 depending on a concrete evaluation.
Thus in cases where a threshold value for the toxic effect is assumed. and a NOAEL or a LOAEL can be identified, the TDI or the LVa;r are obtained by the following calculation:
Soil* |
Soil* |
Air |
Water |
|
oral intake |
dermal contact |
inhalation |
oral intake |
|
| Child, 10 kg | ||||
| average/maximum | 0.2 / 10 g |
1 / 10 g |
10 / 12 m3 |
1 / 2 liter |
| Adult, 70 kg | ||||
| average/maximum | 0.025 / 0.1 g |
0.1 / I g |
20 / 30 m' |
2 / 4 liter |
Risk Evaluation of Contaminated Sites. Miljoprojekt Nr. 123 1990. Ministry of the Environment, Denmark, Danish Environmental Protection Agency. In Danish.7
February 1998 / final
Evaluation of health hazards by exposure to Methyl tertiary-butyl ether
(MTBE)
and estimation of limit values in ambient air, soil and drinking water.
Poul Bo Larsen
The Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
Denmark
1. General description
1.1 Identity
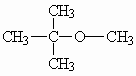
Molecular formula: C5H12O
Structural formula:
| Molecular weight: | 88.15 |
| CAS-no. | 1634-04-4 |
| Synonyms: | tert-Butoxymethane tert-Butyl methyl ether 1,1-Dimethylethyl methyl ether 2-Methoxy-2-methyl propane Methyl-1,1-dimethyl ethyl ether 2-Methyl-2-methoxypropane (2-Methyl-2-propyl)methyl ether MTBE |
1.2 Physical / chemical properties
| Description: | Colourless liquid with characteristic terpene-like odour. |
| Purity: | - |
| Melting point: | -109 ° C |
| Boiling point: | 55.2° C |
| Density: | 0.75 g/ml (at 20° C) |
| Vapour pressure: | 245 mmHg (326 hPa) at 25° C |
| Concentration of saturated vapours: | 322.000 ppm (calculated) (20° C, 760 mmHg) |
| Vapour density: | 3.1 (air = 1) |
| Conversion factor: | 1 ppm = 3.60 mg/m3 20° C 1 mg/m3 = 0.278 ppm 1 atm |
| Flash point: | -28 ° C |
| Flammable limits: | - |
| Autoignition temp.: | - |
| Solubility: | Water: 48 g/100 ml (at 20° C) Very soluble in other ethers, in alcohols and in gasoline. |
| logPoctanol/water: | 0.8 - 1.3 |
| Henry’s constant: | 5.87 x 10-4 (atm x m3)/mole |
| pKa-value: | - |
| Stability: | - |
| Incompatibilities: | - |
| Odour threshold, air: | 0.19 mg/m3 |
| Odour threshold, water: | 0.18 mg/l |
| References: | IPCS (1996), Wibowo (1994). |
1.3 Production and use
In 1994, 6.2 million tonnes was produced in U.S.A. and 3.3 million tonnes in Europe. MTBE is used as an additive in gasoline for increasing the octane content and for enhancing the complete burning of the fuel in order to reduce carbon monoxide and ozone-forming emissions. Typical MTBE concentrations in gasoline are in the range of 7-11 vol%. (IPCS 1996, Wibowo 1994).
In medicine MTBE can be used as an agent to dissolve gallstones (IPCS 1996).
1.4 Environmental occurrence
Air
Due to its high vapour pressure, MTBE will be distributed mainly to the air when released into the environment.
Exposure during refuelling at gas stations is in the range of 0.3-0.5 ppm (1.1-1.8 mg/m3) (Drew 1995). In Finland mean short term exposure of 6.0-7.5 mg/m3 was measured for consumers during tank filling of gasoline containing about 11% MTBE (ECETOC 1997). An average annual exposure level of 0.03-0.05 mg/m3 for maximally exposed persons in the general public has been calculated for areas where gasoline with 15% MTBE content is used during winter (Lucier et al 1995, IPCS 1996). In Fairbanks, Alaska where high levels of MTBE (15 vol%) was used during winter time, the indoor and outdoor level averaged about 0.020 mg/m3 (IPCS 1996).
A geometric mean value of 0.021 mg/m3 was found for air inside cars during an one-hour drive (with a 13-15% MTBE content in gasoline) (IPCS 1996).
For the occupational environment an 8h-average exposure level of up to 31 mg/m3 has been estimated for service stations attendants (Wibowo 1994).
Water
In Denmark MTBE has been found in sub-surface groundwater underneath gasoline stations in a concentration range of 1 µg/l - 500 mg/l, however most of the measurements were in the range of 1-10 mg/l. MTBE has not been found in the deeper primary groundwater (Jensen 1997).
In a study conducted by the United States Geological Survey, detectable concentrations of MTBE was found in 27 % of shallow wells. Of 60 volatile chemicals MTBE was the second most frequently detected chemical. In urban areas the concentration ranged from 0.2 to 23 000 mg/l with a median concentration of 0.6 mg/l (IPCS 1996).
Soil
No data available.
1.5 Environmental fate
Air
Atmospheric MTBE undergoes photooxidation mainly through reaction with photochemically produced hydroxyl radicals. The major initial products are t-butyl formate and 2-methoxy-2-methyl propanal. Further formaldehyde, acetone and CO2 is generated (IPCS 1996).
Soil and water
In surface soil MTBE is due to the high vapour pressure expected to evaporate. As MTBE is of moderate water solubility (the most water soluble constituent in gasoline) wash out with the rain water may be an important elimination route for the deeper soil layer where the evaporation process is suppressed (Larsen 1993).
In conventional tests, MTBE has shown poor biodegradation in soil and water under aerobic as well as anaerobic conditions (IPCS 1996).
No biodegradation of MTBE was found after 60 days in experiments using aquifer soil material as inoculum. After two types of activated sludge as inoculum, no degradation of MTBE occurred after 40 days (Møller Jensen & Arvin 1990 in IPCS 1996).
One study found degradation of MTBE to t-butyl alcohol and CO2 under aerobic conditions using bacterial culture from an industrial sludge sample (Salnitro et al. 1994 in IPCS 1996).
Bioaccumulation
For Japanese carp a log bioconcentration factor of 1.5 has been determined (IPCS 1996).
1.6 Human exposure
The primary route of exposure to humans is through inhalation, as people are exposed to MTBE from air. If a level of 5-10 mg/m3 is anticipated as an average level for the population in the Danish cities, a daily dose of about 100-200 mg is considered a realistic average dose for an adult person with a daily inhalation volume of 20 m3 air. (A level of 5-10 mg/m3 for Danish cities is predicted to be about half the levels of North American cities where 15% MTBE in gasoline were used, see section 1.4).
Further the public may be exposed through the drinking water. As no measurement of MTBE in drinking water have been performed in Denmark it is difficult to make an estimate of the exposure, however the daily doses from drinking water for the general public is considered significantly lower than from inhalation exposure.
2. Toxicokinetics
2.1 Absorption, distribution
Inhalation
MTBE shows a rapid uptake by inhalation exposure in humans. One-hour exposure to 5 mg/m3 MTBE produced a blood concentration of 8.2 and 14.7 mg/l in two volunteers (Prah et al. 1994). For humans exposed to 18, 90 and 180 mg/m3 for 2 hours a respiratory uptake of 32-42% was registered. The blood level of MTBE at the end of the exposure reached levels of 1.3, 6.3 and 12.2 mmole/l (0.11, 0.55 and 1.1 mg/l), respectively (Johanson et al. 1995).
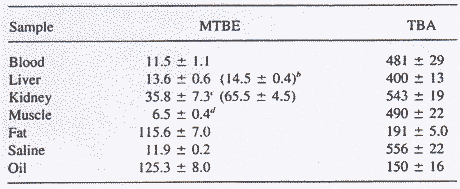
In experimental animals MTBE has been distributed into the various tissues of the body (IPCS 1996). Table 2.1 shows the tissue:air distribution coefficient measured in rats after inhalation exposure to MTBE (exposure level not indicated).
Table 2.1 Tissue:air partition coefficients for MTBE and TBA determined for male F-344-rats (from Borghoff et al. 1996a).
In vitro measurements with human blood showed blood/air distribution coefficients of 17.7 (MTBE) and 462 (t-butyl alcohol, TBA) and water/ air distribution coefficients of 15.2 (MTBE) and 603 (TBA) (Johanson et al. 1995).
Oral intake
In rats 58-81% of a dose of 40 mg/kg was found to be absorbed from the gastrointestinal tract (IPCS 1996).
Dermal contact
Dermal absorption has been demonstrated in studies with rats dermally exposed to MTBE dissolved in water. Forty-eight hours after a six hours exposure to 400 mg/kg under occlusive dressing 35% of the dose was recovered from expired air (20-23%) and urine (12%) (IPCS 1996).
2.2 Elimination
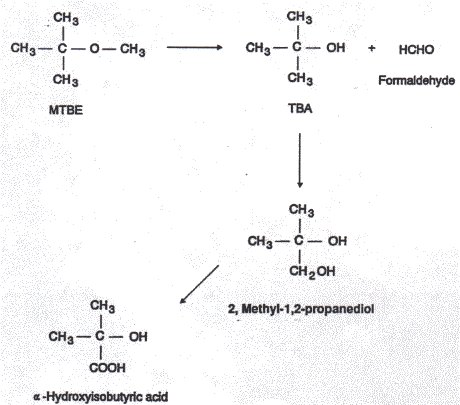
Most of the absorbed MTBE amount of is rapidly eliminated from the body, mainly through expired air as the unchanged compound. MTBE is to some extent metabolised to t-butyl alcohol (TBA) and formaldehyde and oxidised to 2-methyl-1,2-propanediol and a-hydroxy isobuturic acid (IPCS 1996).
Seven hour after one hour exposure to 5 mg/m3 MTBE the concentration in blood from two volunteers had dropped from 8.2 mg/l and 14.7 mg/l to 0.2 and 0.6 mg/l MTBE. In contrast the metabolite TBA gradually increased up to a concentration of 7-10 mg/l (Prah et al. 1994).
AUC values of MTBE and TBA were proportional to exposure levels in humans exposed to 18, 90 and 180 mg/m3 for 2 hours suggesting linear kinetics. MTBE in blood indicated three half-lives of approximately 10 min, 1.5 h and 19 h (Johanson et al. 1995). Of the total inhaled dose of MTBE a fraction of 20-33% were recovered from expired air after the exposure had stopped (total absorption was in the range of 32-42% of inhaled amount). Less than 1% of the absorbed dose of MTBE was excreted as TBA in urine within 24 h, which for the remaining absorbed fraction may indicate further metabolism of TBA as shown in rats (Johanson et al. 1995).
In rats orally dosed with 40 and 400 mg/kg the plasma half-life was found to 0.6 and 0.8 h, respectively. Most of the administrated dose was exhaled as unchanged MTBE but also TBA was detected (2.8 and 1.4% at low and high dose level). In urine major metabolites were further oxidation products of TBA: 2-methyl-1,2-propanediol and a-hydroxyisobutyric acid (IPCS 1996).
Figure 2.1 Metabolism of MTBE, from Miller (1997)
Half-lives of MTBE and TBA in blood of rats have been found to 30 min for MTBE and 1.5-3.5 h for TBA depending of exposure and sex of the rats (Borghoff et al. 1996).
3. Human toxicity
Inhalation
In a chamber exposure study 10 volunteers were exposed during the performance of light physical exercise to 18, 90 and 180 mg/m3 MTBE for 2 hours. Subjective ratings (discomfort, irritative symptoms, CNS effects) and eye measurements (redness, tear film break-up time, conjunctival damage, blinking frequency) and nose measurements (peak expiratory flow, acoustic rhinometry, inflammatory markers in nasal lavage) indicated no or minimal effects of MTBE. However, the ratings of solvent smell increased dramatically by entering the chamber (Johanson et al. 1995).
In another laboratory experiment, 37 volunteers were exposed twice to either clean air or 5 mg/m3 of MTBE for 1 hour. The exposure level was chosen to mimic a realistic exposure level during refuelling a car. Some of the exposed persons experienced bad air quality when exposed to MTBE, however, no increase in symptoms with regard to headache and irritation and other parameters of well-being were observed. No changes in performance was observed in neurobehavioural tests. There were no effects with respect to tear film break, ocular hyperaemia, and in different cytologic markers of ocular and nasal inflammation. In an odour test, a threshold (50% of the persons detecting an odour) in water was found to 0.18 mg MTBE/l (Prah et al. 1994).
In a further study, 43 subjects were exposed to 6 mg/m3 for one hour (the exposure was indicated as a realistic high-level-exposure for commuters). No effects were registered with respect to subjective symptoms, discomfort, various ocular parameters, neurobehavioural performance, and inflammation responses of mucous membranes. The average odour detection level was determined to 0.19 mg/m3 (Cain et al. 1994 cited by IPCS 1996).
In thirteen male volunteers exposed to 0, 90, and 270 mg/m3 for one and three hours, significant effects consisting of feeling of heaviness in the head and mucous membrane irritation was observed at the highest exposure level. No altered performance was found in reaction time test and body sway test (ECETOC 1997).
Several health complaints were reported in Alaska, after the introduction of 15% MTBE gasoline during the winter months. The reportings included symptoms such as headache, eye irritation, burning sensation of the nose or throat, nausea or vomiting, dizziness, sensations of spaciness or disorientation, diarrhoea, fever, sweats, muscle aches, fatigue, difficulty in breathing, skin irritation, and fainting. The effects were of short duration and were reported to be worse at temperatures below -18oC (IPCS 1996).
These reportings are not further described and evaluated in this document because the relation between these unspecific effects and MTBE exposure could not be substantiated. Furthermore, the studies by Prah et al. 1994 and Cain et al. 1994 (described above) were conducted with the aim to disclose relation between the mentioned effects and low level MTBE exposure. However, no effects from MTBE exposure were observed in these well-designed studies. Furthermore, ambient air in cities contain many other respiratory irritants including the photooxidation products of MTBE, and therefore the relationship to MTBE can not be confirmed.
Occupational exposure
Workers adding MTBE to gasoline complained of headaches and nausea. During the process that lasted about 20 minutes maximum values of 6.8-13 mg/m3 MTBE were measured (IPCS 1996). [Elevated levels of gasoline vapours have most probably occurred at the same time].
During barge open loading, MTBE exposure exceeding 360 mg/m3 has caused some workers to complain of odour, nausea, headache and respiratory tract irritation (Wibowo 1994).
In a cross-sectional cohort study, 115 garage workers from a region
using 15% MTBE in the winter season were compared to 122 garage workers in a region where the winter time use of 15% MTBE had ceased 10 weeks before. Active air samples and passive sampling confirmed higher MTBE exposure level of the first group, however, no significant differences were found in the reporting of subjective symptoms (registered by the use of questionnaires) between the groups. Also among a subgroup of fuellers pumping gasoline more than 5 hours per day, no differences were registered (Mohr et al. 1994).
Other routes of exposure
In medicine 1-15 ml of MTBE may be administered by instillation into the gallbladder for dissolving gallstones. Mild complications typically include nausea, vomiting, and pain associated with infusion. Additional effects include signs of transient central nervous system sedation, gastrointestinal irritation, evidence of necrosis in the liver and gall bladder, transient cardiovascular effects such as hyper- or hypotension and palpitations, and transient leucocytosis (IPCS 1996)
4. Toxicity, animal data
4.1 Short term toxicity
Inhalation
In rats, the LC50-value for four-hour inhalation exposure to MTBE is about 85 mg/l. Toxicity signs following exposure were: hyperactivity, eye irritation, salivation, ataxia, weakness, tremors tachypnoea, loss of righting reflex, and unconsciousness. Surviving animal appeared to recover within 24 hours. At necropsy pulmonary hyperaemia was noted (IPCS 1996).
In rats, six hours exposure to 2880 and 14400 mg/m3 resulted in motor activity changes, which also appeared within 10 minutes at 28800 mg/m3. At the two highest dose levels reversible CNS sedation occurred. Survivors killed after a 14 days recovery period had slight to mild lung hyperaemia (IPCS 1996).
In mice, an RD50-value (the dose level that decreases respiratory rate 50%) was found to 16600 mg/m3 (Tepper et al. 1994).
Oral administration
The LD50-value in rats after oral exposure is about 3800 mg/kg. Signs of intoxication were: hypoactivity, muscular weakness, Hyperpnoea, lachrymation, prostration, and death. Recovery was complete at sublethal doses (IPCS 1996).
Repeated gavage administration with doses of 1200 mg/kg/d and higher to rats produced anaesthesia for about 2 h following the administration (Robinson et al. 1990).
In a 28-day oral study a significant increase in mean corpuscular haemoglobin was found down to a dose level of 90 mg/kg/d in female rats. Further a dose-related trend (dose levels of 0, 90, 440 and 1750 mg/kg/day) of increased absolute kidney and liver (primarily female) weight was found. Histopathology showed hyaline droplet formation in the proximal convoluted tubules in the kidneys of mid- and high-dose males (IPCS 1990). LOAEL in this study was 90 mg/kg/day.
Dermal contact
In rabbit the acute dermal LD50-value is > 10200 mg/kg.
Skin irritation
Adverse local effects in a 14 days observation period following 24 hours exposure of rabbits to 6800 and 10200 mg/kg included erythema, oedema, fissuring, and necrosis (IPCS 1996).
Eye irritation
In two separate studies MTBE was judged to cause eye irritation in rabbits after instillation of 0.05 or 0.1 ml of the undiluted liquid. In one study were the irritation was found to be mild a score of 20 out of a max. score of 110 was reached. The effects were reversible (IPCS 1996; the studies were conducted in 1969 and 1980 and thus is difficult to interpret in relation to modern guideline studies).
Eye irritation occurred in rats exposed to vapours at 14400 mg/m3 (IPCS 1996).
In long term studies where mice and rats were exposed to 0, 1440, 10800, and 28 800 mg/m3 the two highest exposure levels produced swollen periocular tissue and spasm of the eyelids (Bird et al. 1997).
Skin sensitisation
MTBE did not induce dermal sensitisation in ten guinea pigs following intradermal injections of 0.1% MTBE every second day for three weeks, with challenge injection of 0.1% MTBE two weeks later (IPCS 1996). [The study is conducted in 1980 and is considered of limited relevance as the study design is not in accordance with guideline studies for skin sensitisation].
4.2 Long term toxicity
Inhalation
In a 13-week vapour inhalation study rats were exposed to 0, 2880, 14400, and 28800 mg MTBE/m3 6h/d, 5d/week. At the two highest dose levels changes in motor activity and body temperature were observed, while further ataxia, depressed body weight gain and increased cortisone levels occurred in the highest dose group. All treatment groups showed increased absolute and relative liver and kidney weights and also increased weight of adrenal glands (primarily males), however, without treatment related microscopic changes in the organs (IPCS 1996). A LOAEL of 2880 mg/m3 could be set from this study.
See also section 4.5.
Oral administration
In a 90-day oral study using dose levels of 0, 100, 300, 900, and 1200 mg/kg/day the relative kidney weight was significantly increased at and above 300 mg/kg/day in female rats and the relative liver, thymic, and cardiac weights showed dose-related increases, statistically significant at 900 mg/kg. In male rats the mean absolute kidney weight was significantly elevated at the two highest dose levels. Microscopic findings in kidneys in high dose male rats were comparable to a2m-globulin nephropathy, otherwise no histopathological findings were noted (Robinson et al. 1990). NOAEL in this study was 100 mg/kg/day.
See also section 4.5.
Dermal contact
No data available.
4.3 Reproductive and developmental effects
In an one-generation study with rats exposed by inhalation to 0, 1080, 4680, or 12240 mg/m3 MTBE no adverse effects on reproduction were registered at the highest dose level (IPCS 1996).
In a two-generation study in which rats were exposed 0, 1440, 10800, or 28800 mg/m3 MTBE a NOEL for reproductive effects was 28800 mg/m3. LOEL for toxicity towards adults and offspring was 10800 mg/m3 (IPCS 1996).
In developmental studies with inhalation exposure of rabbits, rats and mice during gestation, no foetotoxic or developmental effects were noted at exposure levels below the maternal toxicity level. In rabbits NOAEL for maternal toxicity was determined to 3600 mg/m3 and NOAEL for developmental toxicity to >28 800 mg/m3. In mice NOAEL for both maternal and developmental toxicity was found to 3600 mg/m3, and maternal and developmental LOAEL was found to 14 400 mg/m3 due to clinical signs of maternal toxicity and reduced foetal body weight. In rats NOAEL for developmental toxicity was found to 9000 mg/m3, the highest exposure level in the study (IPCS 1996).
4.4 Mutagenic and genotoxic effects
In vitro studies with Salmonella typhimurium and, primary hepatocytes from rats and in vivo studies with Drosophila melanogaster have resulted in negative results. In a forward mutation test using mouse lymphoma cells with metabolic activation MTBE produced a positive and dose-dependent response. The generation of formaldehyde in this special designed test was shown to be the cause of the mutagenic activity. (IPCS 1996).
No positive response was found in an in vivo test with inhalation exposure to mice (2880 - 28800 mg/m3 MTBE 6h/d for 5 days) from which hepatocytes were sampled and examined for DNA repair activity. Nor did MTBE induce micronuclei in bone marrow cells from mice exposed by inhalation (1440 - 28800 mg/m3 MTBE, 6h/d for two days). (IPCS 1996).
4.5 Carcinogenic effects
Inhalation
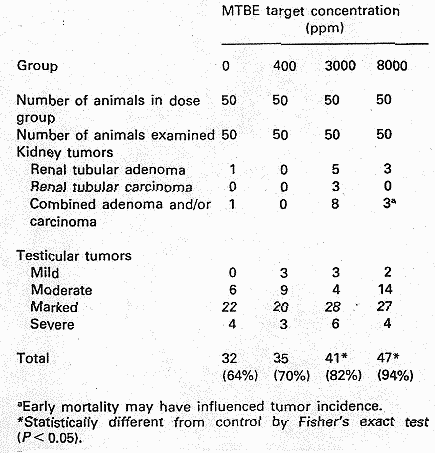
Fischer-344 rats (50 animals/ sex/ exposure level) were exposed up to 24 months to MTBE levels of 0, 1440 , 10800, and 28800 mg/m3 for 6 hours/day, 5 days/week. Increased mortality and decreased mean survival time were observed for male rats from all exposure groups. At the two highest dose levels clinical signs such as hypoactivity, ataxia, lack of startle reflex, swollen periocular tissue, spasm of the eye lids, and salivation were observed in both sexes. In female rats concentration related increases in liver and kidney weight (both absolute and relative) were observed at the two highest dose levels (due to the decrease in survival, statistical analysis could not be performed on organ weights in males for the two highest dose groups). An exposure related increased frequency of chronic nephropathy was observed at all dose levels in males and at the two highest dose levels in female rats. The males were more severely affected than females and nephropathy was the most common cause of death among the males. In males increased incidence of renal tubular cell adenomas and carcinomas was noted at the two highest dose levels (see table 4.1). In mid- and high dose males there was a dose-related increase of interstitial cell adenomas of the testes, see Table 2. (The incidence in the control group was considered low compared to historical data with incidences in the range of 83-88%). The authors suggested a NOEL of 1440 mg/m3 for males and females concerning general toxicity, however, this value may be debated as increased relative kidney weight was observed in male rats at this level. The NOEL for kidney tumours in males was set to 1440 mg/m3 (Bird et al. 1997).
Table 4.1, from Bird et al. (1997).
In CD-1 mice exposed to MTBE levels of 0, 1440 , 10800, and 28800 mg/m3 (50 animals/sex/exposure level) for 6 hours/day, 5 days/week for 18 months increased mortality was found only in males at the highest dose level. Clinical signs at the two highest dose levels were: ataxia, hypoactivity, prostration, lack of startle reflex, stereotypy and spasms of the eye lids. Increased relative liver weight was found in females in the two highest exposure groups. A significant, however not dose-related increase in kidney weight was observed in males from all dose groups and in females at the highest dose level. There was an increased (not significant) frequency of hepatic adenomas and carcinomas in male mice at the highest dose level, and in females, there was a significant increased incidence of hepatocellular adenomas at the highest dose level, see Table 4.2.
Table 4.2, from Bird et al. (1997)
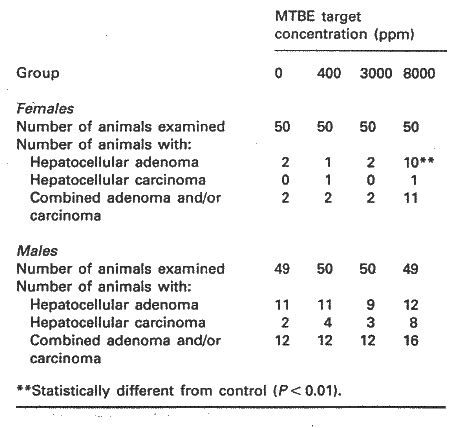
The NOEL for chronic toxicity was by the authors set to 1440 mg/m3 for both male and female mice. With respect to liver tumours the NOEL was set to 10,800 mg/m3 (Bird et al. 1997). Contradictory to this Rudo (1995) also considered the increased incidence of liver carcinomas in male mice as statistically significant.
Oral administration
In an oral study Sprague-Dawley rats (60 animals/sex/dose level) were administered 0, 250, or 1000 mg/kg/day of MTBE in virgin olive oil by gavage 4 days/week for 104 weeks (Belpoggi et al. 1995). High-dose male rats had a higher survival from treatment week 80 than controls (the animals were kept under observation to natural death). In females a treatment-related decrease in survival was observed from treatment-week 16. No evident behavioural changes were noted and no signs of general chronic toxicity were detected by gross and histopathological examination. With respect to oncogenic effects there was a statistically significant increased (p<0.05) incidence of testicular Leydig cell tumours in males in the highest dose group (11 of 32 animals surviving week 96) compared to 2 of 25 animals at the lowest dose level and 2 of 26 animals surviving week 96 in the control group. In female rats, a significant (p<0.01) and dose-related increase in the sum of lymphoma and leukaemia was found at both dose levels (in 12 of 47 animals in the highest dose group and in 6 of 51 animals at the lowest dose level compared to 2 of 58 animals in the control group).
Metabolites tert-butyl alcohol
An oral carcinogenicity study has been conducted with tert-butyl alcohol (TBA) the metabolite of MTBE. Rats were through the drinking water exposed to average daily TBA doses of 0, 85, 195 and 420 mg/kg/day for males and 0, 175, 330, and 650 mg/kg/day for females. Exposure to TBA produced increased incidences of renal tubule adenoma and carcinoma in male rats, and transitional epithelial hyperplasia of the kidney in males and females.
Mice were exposed to average daily TBA doses of 0, 535, 1035, or 2065 mg/kg/day for males and 0, 510, 1015, or 2105 mg/kg/day for females. Exposure to TBA produced a significant increased incidence of follicular cell adenoma of the thyroid in female mice, while a slight increase was observed in males. Further, follicular cell hyperplasia of the thyroid and inflammation and hyperplasia of the urinary bladder in females and males were observed.
(Cirvello et al. 1995).
Formaldehyde
Formaldehyde is the other primary metabolite of MTBE. From experimental animal testing there is sufficient evidence for the carcinogenicity of formaldehyde. Clearest evidence was obtained from inhalation studies in which formaldehyde produced squamous-cell carcinomas of the nasal cavities in rats. In one oral study in which formaldehyde was administered to rats via the drinking water the dosing resulted in increased incidences of leukaemia. (IARC 1995).
Comments
The reporting and the conclusion from the oral MTBE study with rats has been criticised. Firstly, it is noted that the occurrence of Leydig cell tumours is age related, and therefore it may be expected that high dose male rats with a longer survival time than control rats turn out to have a higher incidence of tumours. Secondly, it is criticised that only the combined incidence of leukaemia and lymphoma for female rats is indicated. It is stated that there is little if any scientific reasons to group these two different kinds of tumours, and it is questioned whether the separate incidences of leukaemia and lymphoma would be significantly elevated in the dosed groups (Mennear 1995). Although the critique may be right in these points it seems very questionable that this should explain all the differences compared to the controls.
Further, it is has been argued that the increase in Leydig cell tumours in the inhalation study in rats could rather be explained by an unusual low occurrence in the control group, as the incidence in the dosed group was not elevated significantly compared to historical controls (Mennear 1996). Others however, have stated that more emphasis should be put on concurrent control than historical controls and that the incidence of Leydig cell tumours in the control group in fact should be considered as high. Further a clear dose-response relationship in the study should be recognised as evidence for a substance induced effect. It is considered supportive evidence that the induction of Leydig cell tumours has occurred in two different strains of rats with different background (historical) rates (Rudo 1995).
The exposure period in inhalation study with mice lasted for 18 months, and thus it has been speculated that the induction of hepatocellular adenoma in female mice may had become even more significant if a longer study duration of 24 months had been used for the study (Mennear 1996, Rudo 1995).
MTBE has been shown to bind to the male rats specific protein a2m-globulin and to accumulate in the kidney proximal tubule cells, however, it was found only to be a very mild inducer of a2m-globulin nephropathy. For other chemicals more severe a2m-globulin nephropathy has been shown to be responsible for the development of kidney tumours in male rats. Therefore in the case of MTBE it was suggested that the extra stress due to the a2m-globulin nephropathy may be a possible reason for the development of kidney tumours in the male rats. In females no kidney tumours were observed although MTBE exerted a chronic progressive nephropathy in females as well in males (Borghoff et al. 1996b, Prescott-Mathews et al. 1997).
5. Regulations, limit values
Ambient air
Denmark (C-value): 1 mg/m3 (Larsen 1993).
Drinking water
Denmark: 125 µg/l (Larsen 1993).
EPA-proposal for health advisory for lifetime exposure: 20-200 µg/l (IPCS 1996).
Soil
-
OELs
Denmark: -
Sweden: 50 ppm (180 mg/m3) (Wibowo 1994).
ACGIH, USA: 40 ppm (144 mg/m3) (ACGIH 1996)
Classification
MTBE is not adopted on the List of Chemical Substances (Annex 1).
IARC/WHO
-
US-EPA
A human reference concentration of 3 mg/m3 was derived for MTBE based on a NOAEL of 1453 mg/m3. The exposure level were converted to average continuous exposure level and a total uncertainty factor of 100 was used to account for intraspecies differences, interspecies extrapolation and lack of data (IRIS 1996).
Others
The Secretary´s Scientific Advisory Board on Toxic Air Pollutants, North Carolina examined in 1994 the scientific evidence for the carcinogenicity of MTBE. At the time of the conclusion the data from the oral study with rats was not available, however, the board concluded that there is "some evidence" for carcinogenicity which corresponded to category "C" in the EPA system as a possible human carcinogen. Using the U.S.EPA multistage model for carcinogens on the data from inhalation exposure to experimental animals a human 10-5 lifetime risk was calculated to an average MTBE exposure level in the range of 0.04-0.64 mg/m3. However, it was stated that normally the quantification of risk should not be performed for category C substances (Lucier et al. 1995).
Rudo (1995) recommended on behalf of the Environmental Epidemiology Section, North Carolina to classify MTBE in EPA category B2 as a probable human carcinogen as he evaluated all the carcinogenicity studies including the oral rat study.
6. Summary
Description
MTBE is a colourless liquid with characteristic terpene-like odour.
Use
MTBE is used as an octane enhancing additive in gasoline with a content of 7-11 vol%.
Environment
Due to the high vapour pressure of MTBE (245 mmHg at 25 oC) release to the environment mainly occur through evaporation.
In soil and water MTBE is very persistent towards biodegradation. In soil MTBE may evaporate from surface soil, while wash-out into the ground water may occur from deeper soil layer because of the relatively high water solubility of the substance (48 g MTBE/ l water).
In air MTBE undergo photochemical degradation.
Human exposure
Inhalation is considered the most important exposure route for human MTBE exposure. Short term exposure levels of 6-7.5 mg/m3 MTBE have been measured during refuelling cars at tank stations. In Fairbanks in Alaska where 15% MTBE gasoline is used during winter time an average outdoor and indoor level of 0.020 mg/m3 was measured. Based on such data a level for the ambient air in urban areas in Denmark could preliminary be estimated to 0.005-0.010 mg/m3.
Toxicokinetics
MTBE is rapidly taken up by inhalation exposure in humans with a retention of 30-40%. From animal studies it has been shown that MTBE is easily absorbed after oral and dermal exposure.
MTBE is rapidly eliminated from the body, mainly through expired air as the unchanged compound. MTBE is to some extent metabolised to tert-butyl alcohol (TBA) and formaldehyde. In the organism TBA has a longer half-life than MTBE. In humans elimination of MTBE from blood occurred in three phases with half-lives of 10 minutes, 1.5 hour and 19 hours.
Human toxicity
For humans an average odour detection limit of 0.19 mg/m3 has been detected in air and 0.18 mg/l in water. From the occupational environment and from areas where MTBE content in gasoline was increased up to 15 vol% there have been several reportings of irritation of eyes and respiratory tract, headache, nausea, dizziness and other unspecific symptoms. However, no causal relationship to MTBE exposure could be derived from these data, as the persons have been exposed to gasoline vapours and exhaust emission as well. In order to elucidate the effect of MTBE two controlled laboratory experiments with humans have been conducted. In these studies a one hour exposure to 5 and 6 mg/m3 MTBE, respectively, did not result in any subjective symptoms other that sensation of odour.
A third chamber study with exposure up to 180 mg/m3 MTBE for 2 hours was also negative with respect to the induction of any irritation symptoms, while a study using an exposure level of 270 mg/m3 resulted in mucous membrane irritation and feeling of heaviness in the head.
Animal toxicity acute effects
In animals the oral LD50-value for rats is about 3800 mg/kg. The LC50-value for rats after 4 hours exposure is about 85 000 mg/m3.
In mice an RD50-value (the concentration that produce a 50% decrease in respiratory rate) of 16 600 mg/m3 was registered indicating respiratory tract irritation of the substance. Exposure to 10 800 mg/m3 has resulted in swollen periocular tissue and spasm of the eyelids.
CNS effects have been observed in connection with inhalation exposure: hypoactivity, sedation, ataxia, weakness, loss of righting reflex, lack of startle reflex, tremors and unconsciousness. Changes in motor activity occurred in rats down to an exposure level of 2880 mg/m3. Oral administration of 1200 mg/kg to rats produced anaesthesia that lasted for up to 2 hours.
The kidney and the liver has been demonstrated to be the target organ in short term studies with repeated exposure. From inhalation studies a LOAEL of 2880 mg/m3 could be set based on increased relative liver and kidney weights in rats. From studies with oral administration a NOAEL of 100 mg/kg/d and a LOAEL of 300 mg/kg/d in rats could be set due to these effects. (A LOAEL of 90 mg/kg/d was found in a 28-day oral study with rats due to increased value in mean corpuscular haemoglobin, however this effect could not be verified from other studies).
chronic effects / carcinogenicity
From long term/ carcinogenicity studies a NOAEL of 1440 mg/m3 could be set for female rats based on effects on liver and kidneys at the higher exposure levels (10800 and 28800 mg/m3). A LOAEL of 1440 mg/m3 could be set for males due to slight increase in nephropathy (maybe related to a2m-globulin accumulation). In mice a NOAEL of 1440 mg/m3 could be set.
Carcinogenicity studies using inhalation exposure to 0, 1440, 10800, and 28800 mg/m3 were conducted with rats and mice. In male rats, exposure to 10800 mg/m3 and 28800 mg/m3 produced increased incidences of renal tubular cell adenomas and carcinomas and dose related increase in interstitial cell (Leydig cell) adenomas of the testes. In mice, 28800 mg/m3 produced an increased incidence of hepatocellular adenomas in the female animals.
It has been suggested that the occurrence of renal tubular cell tumours in male rats could be the follow of a2m-globulin nephropathy as accumulation of a2mglobulin has been demonstrated after MTBE exposure to male rats. However, MTBE has only been shown to be a very mild inducer of a2m globulin nephropathy, and therefore the mechanism behind the tumourigenic effects in the male rat kidney seems not to be fully elucidated.
In a study with gavage administration of 0, 250, and 1000 mg/kg/day to rats a statistically increased incidence of testicular Leydig cell tumours occurred at the highest dose level. However, longer life-time of high dose male rats may to some extent explain this finding as the occurrence of Leydig cell tumours is age related. In female a significantly and dose-related increased in the sum of lymphoma and leukaemia was observed at both dose levels.
Reproductive and developmental effects
MTBE has not produced any reproductive effects in one single - and one two-generation test with rats. From developmental studies with rats mice and rabbits no indication of developmental or teratogenic effects were found below maternal toxic dose levels. NOAEL for maternal toxicity was found in the range of 3600 to 10800 mg/m3 and LOAEL for maternal toxicity and foetal toxicity (reduced foetal body weight) was found to 14 400 mg/m3.
Mutagenic and genotoxic effects
MTBE has been found negative in in vivo mutagenicity tests for DNA repair and micronuclei formation in mice after inhalation exposure. In vitro bacterial assays and an assay with rat hepatocytes were negative. One in vitro test with a mouse lymphoma cell line with metabolic activation resulted in positive result which was shown to be due to the formation of formaldehyde.
Carcinogenicity
See above.
7. Evaluation
MTBE has shown to cause tumourigenic/ carcinogenic effects in experimental animals as described above. The effects occur at dose levels of considerable organ toxicity. As MTBE was not shown to be genotoxic the tumourigenic effects are considered mediated through non-genotoxic mechanism secondary to organ toxicity. Therefore, a threshold for the tumourigenic effect is anticipated.
From a 90-day oral study a NOAEL of 100 mg/kg/d has been determined as higher dose levels (300 and 900 mg/kg/d) produced kidney toxicity in both male and female rats.
From inhalation studies a NOAEL of 1440 mg/m3 for exposure periods of 6 hours/day, 5 days/week for up to two years was found for female rats (due to occurrence of a2mglobulin accumulation in male rats kidney tubules cells it is not possible to identify a relevant NOAEL for risk assessment in male rats concerning effects on the kidney).
With respect to eye and respiratory tract irritation, studies with human volunteers did not find any signs of irritation up to 180 mg/m3 (the highest level tested). However, the subjective sense of odour from MTBE should be considered when establishing limit values for MTBE in air and water due to the potent terpene-like odour from the substance. In that respect odour detection limits (50% detection limit) of 0.19 mg/m3 and 0.18 mg/l have been determined for MTBE in air and water, respectively.
8. TDI, health based limit values
8.1 TDI
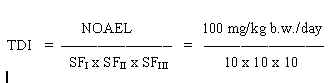
0,1 mg/kg b.w./day
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 10 because the NOAEL was established on a 90-day study and to consider the severity of the effects (cancer) that may follow exposure to a toxic level.
Allocation
The general population is considered predominantly to be exposed to MTBE from air. Therefore, only 10% of the TDI is allocated to ingestion of soil and drinking water, respectively.
8.2 Limit value in soil
Based on the TDI of 0.1 mg/kg b.w. per day and assuming a daily ingestion of 0.2 g soil for a child weighing 10 kg (wchild), a limit value is calculated:
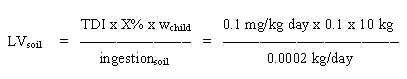
= 500 mg/kg soil
Such a value seems unrealistic high due to the odour potency of the substance, therefore a limit value for soil should rather be based on the evaporation of MTBE from the soil.
8.3 Limit value in drinking water
Based on the TDI of 100 mg/kg b.w. per day and assuming a daily ingestion of 2 litres of drinking water for an adult weighing 70 kg (wadult), a limit value is calculated:
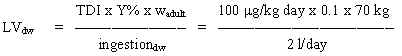
= 350 mg/l
Such a limit value for water seems unrealistic high due to the odour potency of the substance, therefore, a limit value for drinking water
should be based on the odour detection limit in water.
8.4 Limit value in air
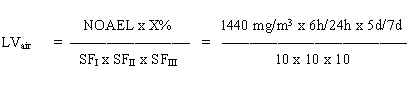
= 0.26 mg/m3
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 10 because the severity of the effect (cancer) that may follow the toxic effects.
This value is just above the experimental 50% detection odour limit for humans. Therefore, a limit value for MTBE in air should be based on the odour detection limit.
9. C-value
9.1 Quality criteria in soil
A limit value of 500 mg/kg has been calculated based on ingestion of soil by children.
However, MTBE has a low odour threshold in air. Therefore, to account for discomfort from the odour due to evaporation from the soil, the C-value should not be exceeded in the air above soil surface.
Quality criteria
Quality criterion for porous air in soil: 0.3 mg/m3 (based on odour).
9.2 Quality criteria in drinking water
A limit value of 350 mg/l has been calculated based on intake of drinking water.
However, MTBE has a low odour threshold in water. Therefore, to take into account the discomfort from the odour, a quality criterion of 30 mg/l is proposed. This criterion is calculated based on the 50% odour detection limit in water (180 mg/l) and according to the calculation method of the Danish Environmental Protection Agency (conversion of 50% detection value to a 10% detection value = 50% value in mg/m3 x 0.18/ standard deviation for the observations).
Quality criteria
Quality criterion: 30 mg/l (based on odour).
9.3 C-value
A toxicologically based limit value of 0.26 mg/m3 has been calculated. However, MTBE has a low odour threshold in air. Therefore, to account for discomfort from the odour, a limit value of 0.03 mg/m3 is calculated based on the 50% odour threshold in air (0.19 mg/m3) and according to the calculation method of the Danish Environmental Protection Agency (conversion of 50% detection value to a 10% detection value = 50% value in mg/m3 x 0.18/ standard deviation for the observations).
For substances, for which odour is the limiting factor, the C-value is set at the odour limit value. Therefore, a C-value of 0.03 mg/m3 is proposed. MTBE should be placed in main group 1, as the substance has shown to be carcinogenic in experimental animals.
C-value
0.03 mg/m3 (based on odour), Main Group 1.
10. References
ACGIH (1996). TLVs and BEIs, threshold limit values for chemical substances and physical agents biological exposure indices.
Belpoggi F, Soffritti M and Maltoni C (1995). Methyl tertiary-butyl ether (MTBE) - A gasoline additive - causes testicular and lymphohaematopoetic cancers in rats. Toxicol Ind Health 11, 119-149.
Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ and Andrews LS (1997). Oncogenicity studies of inhaled methyl tertiary-butyl ether (MTBE) in CD-1 mice and F-344 rats. J Appl Toxicol 17, S45-S55.
Borghoff SJ, Murphy JE and Medinsky MA (1996a). Development of a physiologically based pharmacokinetic model for methyl tertiary-butyl ether and tertiary-butanol in male Fischer-344 rats. Fund Appl Toxicol 30, 264-275.
Borghoff SJ, Prescott-Mathews JS and Poet TS (1996b). The mechanism of male rat kidney tumours induced by methyl tert-butyl ether and its relevance in assessing human risk. CIIT Activities, Chemical Industry Institute of Toxicology 16, 1-8.
Cain WS, Leaderer BP, Ginsberg GL, Andrews LS, Cometto-Müniz JE, Gent JF, Buck, M, Berglund LG, Mohsenin V, Monahan E and Kjaergaard S (1994). Human reactions to methyl tertiary-butyl ether (MTBE). Unpublished data from John B Pierce Laboratory, New Haven, CT 06519. (Quoted by IPCS 1996).
Cirvello JD, Radovsky A, Heath JE, Farnell DR and Lindamood C (1995). Toxicity and carcinogenicity of t-butyl alcohol in rats and mice following chronic exposure in drinking water. Toxicol Ind Health 11, 151-165.
ECETOC (1997). Methyl tert-butyl ether, health risk characterisation. Technical report no 72, European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, 126 p.
IARC (1995). Formaldehyde. In: Wood dust and formaldehyde, IARC monographs on the evaluation of carcinogenic risks to humans. International Agency for Research on Cancer/ WHO, vol 62, 217-362.
IRIS (1997). Methyl tert-butyl ether. In: Integrated Risk Information System. Database quest, last revision September 1993. US-EPA.
IPCS (1996). Methyl tertiary-butyl ether. Environmental Health Criteria (draft). World Health Organization, International Programme on Chemical Safety, Geneva. pp 150 (Quoted with permission of the IPCS).
Jensen MM (1997). Personal communication, the Division of contaminated soil, Danish EPA.
Johanson G, Nihlén A and Löf A (1995). Toxicokinetics and acute effects of MTBE and ETBE in male volunteers. Toxicology Letters 82/83, 713-718.
Larsen (1993). Gasoline and diesel fuel contaminated sites - a toxicological evaluation -. Environmental Project no. 223, Danish EPA. pp 205. (in Danish with English summary).
Lucier G, Genter MB, Lao YJ, Stopford W and Starr T (1995). Summary of the carcinogenicity assessment of MTBE conducted by the Secretary´s Scientific Advisory Board on toxic air pollutants. Environ Health Perspect 103, 420-422.
Mennear JH (1995). MTBE: not carcinogenic. Environ Health Perspect 103, 985-986.
Mennear JH (1996). MTBE: Possible human carcinogen or not. Proceedings from presentation at the Toxicology Forum 19-21 February, Capital Hilton, Washington, 139-151.
Miller MJ, Ferdinandi ES, Klan M, Andrews LS, Douglas JF and Kneiss JJ (1997). Pharmacokinetics and disposition of methyl t-butyl ether in Fischer-344 rats. J Appl Toxicol 17, S3-S12.
Mohr SN, Fiedler N, Weisel C and Kelly-McNeil K (1994). Health effects of MTBE among New Jersey garage workers. Inhal Toxicol 6, 553-562.
Møller Jensen and Arvin E (1990). Solubility and degradability of the gasoline additive MTBE, methyl tert-butyl ether, and gasoline compounds in water. In: Contaminated Soil ´90 (Eds. Arendt F, Hinsewald M, & van der Brink WJ), 445-448.
Prah JD, Goldstein GM, Devlin R, Otto D, Ashley D, House D, Cohen KL and Gerrity T (1994). Sensory, symptomatic, inflammatory, and ocular responses to the metabolism of methyl tertiary butyl ether in a controlled human exposure experiment. Inhal Toxicol 6, 521-538.
Prescott-Mathews JS, Wolf DC, Wong BA and Borghoff SJ (1997). Methyl tert-butyl ether causes a2m-globulin nephropathy and enhanced renal cell proliferation in male Fischer-344 rats. Toxicol Appl Pharmacol 143, 301-314.
Robinson M, Bruner RH and Olson GR (1990). Fourteen- and ninety-day oral toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley rats. J Am Coll Toxicol 9, 525-540.
Rudo KM (1995). Methyl tertiary butyl ether (MTBE) - evaluation of MTBE carcinogenicity studies. Toxicol Ind Health 11, 167-173.
Salanitro JP, Diaz LA, Williams MP and Wisniewski HL (1994). Isolation of a bacterial culture that degrades methyl-t-butyl ether. Appl Environ Microbiol 60, 2593-2596.
Tepper JS, Jackson MC and McGee JK (1994). Estimation of respiratory irritancy from inhaled methyl tertiary butyl ether in mice. Inhal Toxicol 6, 563-569.
Wibowo AAE (1994). Methyl-tert-butyl ether. Arbete och Hälsa no. 1994:22, pp 21.
May 1999 / final
Evaluation of health hazards by exposure to
Formaldehyde
and estimation of limit values in ambient air, soil and drinking water.
Poul Bo Larsen
Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
Denmark
1. General description
1.1 Identity
| Molecular formula: | CH2O |
| Structural formula: | H2- C = O |
| Molecular weight: | 30.03 |
| CAS-no.: | 50-00-0 |
| Synonyms: | Formic aldehyde Methanal Methylene oxide Methylaldehyde Oxymethylene Oxomethane |
1.2 Physical / chemical properties
| Description: | Formaldehyde is a colourless, flammable, reactive and readily polymerised gas at normal temperature and pressure. A gas with a pungent, irritating odour. |
| Melting point: | -92 ° C |
| Boiling point: | -21 ° C |
| Density: | 0.815 g/ml (at 20° C) |
| Vapour pressure: | 3284 mmHg (438 kPa) at 20° C |
| Vapour density: | 1.04 (air = 1) |
| Conversion factor: | 1 ppm = 1.25 mg/m3 20° C 1 mg/m3 = 0.801 ppm 1 atm |
| Solubility: | Water: Very soluble. Very soluble in ethanol and diethyl ether. |
| logPoctanol/water: | 0.35 |
| Henry’s constant: | 3.27 x 10-7 (atm x m3)/mole at 20° C |
| Stability: | In moist air and in concentrated solutions at room temperature, polymerisation takes place to form paraformaldehyde. |
| Incompatibilities: | Reacts explosively with peroxides, nitrogen oxide and performic acid; can react with hydrogen chloride or other inorganic chlorides to form bis(chloromethyl)ether. |
| Odour threshold air: | 0.03 mg/m3 (10-percentile, odour detection). 0.18 mg/m3 (50-percentile, odour detection). |
| Odour threshold water: | 49.9 mg/l (average) 0.8-102 mg/l (range) |
| References: | IARC (1995), HSDB (1996), IPCS (1989), Verschueren (1983), WHO (1998). |
1.3 Production and use
Formaldehyde is produced commercially by the catalytic oxidation of methanol. The widest use is in the production of resins with urea, phenol and melamine. Formaldehyde-based resins are used as adhesives and impregnating resins in the manufacture of particle-board, plywood, furniture, and other wood products. The resins are also used in the textile, leather, rubber, and cement industries. Further, formaldehyde is used in the chemical industry as an intermediate in a variety of chemical synthesis. Formaldehyde itself is used for preservation and disinfection e.g. by embalming of biological specimens. It is used as an antimicrobial agent in many cosmetics products e.g. soaps, shampoos, hair preparations deodorants, lotions, and make-ups. (IARC 1995).
1.4 Environmental occurrence
Formaldehyde is ubiquitous in the environment; it is an important endogenous substance that occurs in most life forms. In humans, as well as in other animals, formaldehyde is an essential metabolic intermediate in all cells in the biosynthesis of purines, thymidine, and certain amino acids. (IARC 1995).
Air
Formaldehyde is formed by photooxidation of hydrocarbons in the troposphere where naturally occurring methane is the most important source for the production. The non-urban background level of formaldehyde is <1 mg/m3 with a mean of about 0.5 mg/m3.
Formaldehyde together with other aldehydes contributes about 12% of the volatile organic compounds in automobile exhaust from gasoline cars; diesel exhaust contains about 5% aldehydes (Larsen et al. 1997). Levels in urban areas with anthropogenic hydrocarbon and aldehyde emissions from traffic are reported to 1-20 mg/m3 (IARC 1995).
In streets with dense traffic in Copenhagen, average and maximum levels of 4.3 and 8.3 mg/m3 have been reported during a winter period (Granby et al. 1997). Higher levels would be expected during summer months.
Water
Formaldehyde levels in rainwater are reported to 0.1-0.2 mg/kg. Drinking water normally contains <0.1 mg/l (IPCS 1989). Formaldehyde in drinking water is mainly formed by oxidation of natural organic (humic) matter during ozonation and chlorination or it enters the water from polyacetal plastic fittings. Up to 30 mg/l has been found in ozonated drinking water (WHO 1996).
Soil
No information is available concerning formaldehyde in soil.
Foodstuffs
There is some natural formaldehyde in raw food. Fruits and vegetables typically contain 3-60 mg/kg (e.g. pear: 60 mg/kg and apple: 17 mg/kg); milk and milk products about 1 mg/kg; meat and fish 6-20 mg/kg and shellfish 1-100 mg/kg. (IARC 1995, IPCS 1989).
1.5 Environmental fate
Air
In air, formaldehyde photolyses and reacts rapidly with reactive free radicals. The half-life in sunlight is a few hours. Because of high water solubility formaldehyde may be transferred to and eliminated with the rain. (HSDB 1996).
Water
When released into water, formaldehyde will biodegrade to low levels within few days. Due to a low Henry´s Law constant of 3.7 x 10-7 atm m3/mole volatilisation from water should not be significant. Little adsorption to sediment would be expected to occur. In nutrient-enriched sea-water there is a long lag period (approximately 40 hours) prior to measurable loss of formaldehyde by presumably biological processes. The fate of formaldehyde in ground water is unknown. (HSDB 1996).
Soil
Formaldehyde is easily biodegradable in soil. The adsorption coefficient to soil is very low, thus mobility is high and leaching may easily occur (IPCS 1989).
1.6 Human exposure
Humans are mainly exposed to formaldehyde by inhalation of ambient and indoor air containing formaldehyde. Indoor air may contain much higher levels than ambient air due to evaporation of formaldehyde from furniture, paint and building constructions. Levels between 10 and 1000 mg/m3 have been reported. The contribution from various atmospheric environments to the average human daily intake has been calculated to be 0.02 mg/day for outdoor air, 0.5-2 mg/day for indoor conventional buildings, and up to 1-10 mg/day for buildings with sources of formaldehyde. Smoking 20 cigarettes per day contributes with an additional exposure of about 1 mg formaldehyde. (IPCS 1989).
The quantity of formaldehyde ingested in food depends on the composition of the meal and may, for an average adult, range from 1.5 to 14 mg/day (IPCS 1989).
Humans are dermally exposed to formaldehyde in connection with use of various cosmetic products containing formaldehyde. The absorption from dermal exposure is estimated to be negligible (IPCS 1989).
2. Toxicokinetics
2.1 Absorption, distribution
The endogenous concentration of formaldehyde in the blood of human subjects not exposed to formaldehyde is reported to 2.6 mg/kg as the sum of free and reversible bound formaldehyde (IARC 1995).
Inhalation
In experimental animals almost 100% of inhaled formaldehyde is retained by the tissues of the respiratory tract. The retention takes place almost entirely in the nasal passages of rats. In rhesus monkeys retention occurs primarily in the nasal passages but also in the trachea and proximal regions of the major bronchi. The efficiency by which formaldehyde is retained was found to be determined by the nasal anatomy, as structures causing more narrow and complex air flow enhances retention by the surface of the nasal mucosa (IARC 1995).
At identical exposure levels mice were found to receive a lower effective dose at the target tissue in the nasal cavities than rats as the mice reacted with a greater reduction in minute ventilation as a response from sensory irritation of the respiratory tract. The less intense exposure of mice were further verified by smaller increases in cell proliferation in the nasal mucosa compared to rats. The authors suggested that these findings could explain why higher formaldehyde exposure levels were needed to induce the same degree of toxic effects (e.g. nasal tumours) in mice as seen in rats at lower levels (Chang et al. 1983).
In rats and humans increases in blood concentration of formaldehyde were not detected after formaldehyde exposure because of high reactivity and rapid metabolism (IPCS 1989). Thus, no changes in blood formaldehyde content were found in rats exposed for 2 hours to 17.6 mg/m3 or in monkeys exposed for 6 hours/day, 5days/week during four weeks to 7.3 mg/m3 (IPCS 1995).
Oral intake
In experimental animals formaldehyde is rapidly and almost completely absorbed after oral exposure. A substantial part of radioactivity from radiolabelled formaldehyde was found distributed in the carcass because of metabolic incorporation of the radiolabelled carbon (IARC 1995).
There is no data showing that formaldehyde per os give rise to increased formaldehyde levels in blood, so formaldehyde is most probably instantaneously converted by reaction with macromolecules or by metabolic processes.
Dermal contact
In monkeys less than 1% of a dose of an aqueous formaldehyde solution applied to skin was absorbed (amount of radioactivity gained from expired air), while 10% was bound to the exposed skin area. In rats about 30% absorption of formaldehyde from skin application has been registered. (IARC 1995).
There is no data showing that formaldehyde after dermal exposure gives rise to increased formaldehyde levels in blood, so formaldehyde is most probably instantaneously converted by reaction with macromolecules at the skin surface or by metabolic processes during penetration of the skin layer.
2.2 Elimination
Metabolism
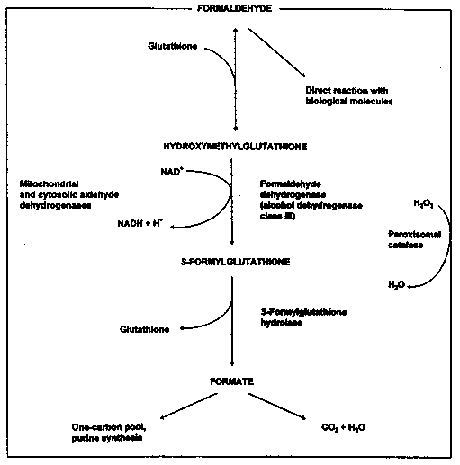
As human erythrocytes contain formaldehyde dehydrogenase and aldehyde dehydrogenase absorbed formaldehyde may be rapidly metabolised (IARC 1995). The metabolism of formaldehyde is illustrated in Figure 2.1.
Figure 2.1 Metabolism and fate of formaldehyde (from IARC 1995).
Formaldehyde can react with macromolecules at the site of entry. DNA-protein cross-links have been detected in tissues exposed directly to formaldehyde, but not in tissues remote from the absorption site. Formaldehyde subjected to metabolism are incorporated into macromolecules via one-carbon path-ways or are eliminated in the expired air (CO2) and urine. (IPCS 1989).
Excretion
In rats about 40% of the inhaled dose of radiolabelled formaldehyde (0.8 and 16 mg/m3 for 6 hours) was eliminated as CO2 over a 70-hour period; 17% of the radioactivity was excreted in the urine; 5% was eliminated in faeces while 35-39% remained in tissues and carcass (IARC 1995).
Using radiolabelled formaldehyde 40% of an oral dose in rats was found to be eliminated with the expiratory air as CO2. Ten percent was eliminated with urine and 1% with faeces (IARC 1995).
Seventy-two hours after dermal exposure to rats, 6.6% of the applied dose of formaldehyde was eliminated with the urine, while less than 1% was recovered from expiratory air (IARC 1995).
3. Human toxicity
3.1 Short term toxicity
An immense amount of data exists concerning acute effects from formaldehyde exposure. However, there is a variability in the quality of the studies and reportings and due to big differences in individual sensitivity and in the effect concentrations observed from the different studies, it is from several expert groups deemed almost impossible to point out single studies for identifying the absolute NOAEL and LOAEL value, from which recommendations can be made. Thus a more overall view of the data has been used to establish dose-response and dose-effect relationships (IPCS 1989, ACGIH 1991, WHO 1998, Paustenbach 1997). This is reflected in the following description of the effects of formaldehyde which more focuses on the conclusions from the expert groups rather than detailed evaluation of the specific studies and reportings.
Vapour exposure odour
IPCS (1989) indicates a level between 0.06 mg/m3 and 0.22 mg/m3 as odour threshold, while levels of 0.031-0.177 mg/m3 (non-smokers) and 0.025-0.58 mg/m3 (smokers) were reported by IARC (1995). In the WHO (1998) evaluation, 0.03, 0.18, and 0.6 mg/m3 were reported as the 10-, 50- and 90-percentiles for the odour detection threshold.
Irritative effects on the respiratory tract and eyes
The common effects related to formaldehyde exposure are various symptoms caused by irritation of the mucosa in the eyes and the upper airways. The symptoms reported are headache, irritation/ burning sensation of the eyes, sore throat and annoyance because of smell. In the non-industrial indoor environment the sensory reactions are typical effects, but there appear to be large individual differences in the normal population and among hyper-reactive people.
In the evaluation of IPCS (1989) it was concluded that no absolute irritation threshold could be set for formaldehyde. It was acknowledged that sensory irritation for the eyes were reported at 0.06 mg/m3 and for the respiratory tract at 0.12 mg/m3. Overall it was recommended that the level of formaldehyde in ambient air should not exceed 0.1 mg/m3. To account for hypersensitive people without immunological signs it was further recommended that formaldehyde concentrations should not exceed 0.01 mg/m3.
ACGIH (1991) concluded that formaldehyde levels of 0.25-0.5 ppm (0.3-0.6 mg/m3) would be troublesome for up to 20% of a population and that 10-20% would react acutely to levels below 0.25 ppm as some studies have reported mucous membrane irritation at concentrations as low as 0.1 ppm (0.1 mg/m3). On the overall data ACGIH recommended a threshold limit value (as ceiling value) for formaldehyde of 0.3 ppm (0.37 mg/m3) in the occupational environment.
WHO (1998) tabulates the following dose effect relationship:
Table 3.1 Effects of formaldehyde after short term exposure
| Concentration, range or average, mg/m3 |
Exposure duration | Health effects in general population |
| 0.1-3.1 | single and repeated exposure | throat and nose irritation threshold |
| 0.6-1.2 | single and repeated exposure | eye irritation threshold |
| 0.5-2 | 3-5 hours | decreased nasal mucous flow rate |
| 2.4 | 40 min. on 2 successive days with 10 min. moderate exercise on second day | post exposure (up to 24 hours) headache |
| 2.5-3.7 | - | biting sensation in eyes and nose |
| 3.7 | single and repeated exposure | decreased pulmonary function only at heavy exercise |
| 5-6.2 | 30 min. | tolerable for 30 min. with lachrymation |
| 12-25 | - | strong lachrymation, lasting for 1 hour |
| 37-60 | - | pulmonary oedema, pneumonia, danger to life |
| 60-125 | - | death |
In the overall evaluation of WHO (1998) an irritation threshold of 0.1 mg/m3 after short term exposure for the general population is noticed, while a progression of symptoms and effects occur at levels above 1.2 mg/m3. To prevent significant sensory irritation an air quality guideline of 0.1 mg/m3 is proposed.
Paustenbach et al. (1997) in a recent evaluation of a panel of experts (The Industrial Health Foundation Panel) re-evaluated the scientific basis for a formaldehyde dose-response relationship and for the setting of an occupational exposure limit. The panel found that the most reliable data in which effects and response rates were related to specific formaldehyde exposure levels were human data from laboratory chamber exposure. From these studies ten key studies were identified by the panel. Furthermore, six studies with occupational exposure and two studies from community surveys were considered important for the setting of an occupational exposure limit.
The panel concluded that eye irritation is the most sensitive effect related to formaldehyde exposure. From the dose-response relationship obtained by linear least-square regression of the data from the key studies, the panel estimated that 1-5% of a population would feel transient eye irritation at a level of 0.3 ppm (0.37 mg/m3) and 5-25% at a level of 0.5-1.0 ppm (0.6-1.2 mg/m3). A level of 0.1 ppm (0.12 mg/m3) was judged to prevent irritation in virtually all persons. Furthermore, it was stated that significant irritation due to formaldehyde exposure in most studies does not occur until an exposure level of 1.0 ppm (1.2 mg/m3) is reached. Based on this the panel recommended an occupational exposure limit of 0.3 ppm (0.37 mg/m3) as an 8-hour average and a ceiling value of 1 ppm (1.2 mg/m3) to avoid irritation.
pulmonary effects
Inhalation studies using formaldehyde levels up to 2.4 mg/m3 did not cause any effects on lung function parameters in human volunteers, however, at higher levels (above 6 mg/m3) pulmonary effects and effects from the lower airway are likely to occur (IPCS 1989).
sensitivity
Experimental data indicate great variability between individuals in sensitivity towards the effects of formaldehyde exposure. However, the data do not indicate that asthmatic persons seem to be more sensitive compared to the non-asthmatic persons (Paustenbach et al. 1997, IARC 1995, IPCS 1989). Nevertheless, asthma-like symptoms have been reported at irritant concentrations from occupational studies (IPCS 1989).
Oral intake
Ingested formaldehyde solution has resulted in corrosive injury in the stomach. Lethal outcome after formaldehyde ingestion has occurred after the ingestion of a few drops to 89 ml of concentrated solution (i.e. about 40% aqueous solution). The largest quantity survived is reported to 120 ml. After ingestion of 60-90 ml of a 40% solution death was associated with pronounced injury in the oesophagus and the gastrointestinal tract: all organs and tissues in contact with the stomach were dark (chocolate brown) and hardened to a depth of about 8 mm (IPCS 1989).
Dermal contact
Contact may cause burns to skin (HSDB 1996).
The concentration of aqueous formaldehyde solution causing irritant reactions has not been specifically determined but a 1% solution applied under occlusive dressing is considered to produce irritant response in approximately 5% of the population. Cosmetics containing a formaldehyde concentration of 0.2% as a preservative and nail hardeners containing at least 5% formaldehyde did not provoke toxic or irritative reactions on normal skin (IPCS 1989).
Eye contact
Contact with the eye may cause burns. Depending on the formaldehyde concentration, aqueous solutions in contact with the eye have caused injuries ranging from severe, permanent corneal opacification and loss of vision to minor transient injury or discomfort (Grant 1986).
A splash of 0.2% solution of formaldehyde has been reported to cause irritation with stinging and hyperaemia, but without permanent injury. One drop of a 40% solution in an eye which was instantly washed resulted in pain after 6 hours and corneal opacity that still lasted after 6 months. Other cases with eye splash of 40% formaldehyde solution have resulted in blindness and loss of the eye (Grant 1986).
(With respect to eye irritation from vapour exposure see the section vapour exposure).
Sensitising effects - inhalation
There are only few reports on sensitisation associated with inhalational exposure to formaldehyde. In one report including 230 persons with asthma like symptoms inhalational provocation test with formaldehyde identified 12 persons with specific positive response. Several other studies, however, with less persons involved, also report of positive provocation tests with formaldehyde (IPCS 1989). Patterson et al. (1989) and Grammer et al. (1991) from their studies with persons suffering from respiratory symptoms in relation to formaldehyde exposure found no evidence for immunologically mediated asthma from formaldehyde exposure. Patterson et al. (1989) concluded that there is no evidence for formaldehyde being an inhalational antigen, while Grammer et al. (1991) judged respiratory sensitisation from formaldehyde to be extremely rare, if at all existing.
Also Bardana & Montanaro (1991) in their review on immunologic effects concluded that no data until now have proven the induction of IgE-mediated respiratory tract symptoms caused by inhalational exposure to formaldehyde. Only on rare occasions were formaldehyde at high exposure levels found to induce bronchial asthma.
- dermal contact
Formaldehyde solution is a primary skin-sensitising agent inducing allergic contact dermatitis (type IV, T-cell mediated delayed hypersensitivity reaction); it may induce immunological contact urticaria (type I, perhaps IgE mediated, immediate hypersensitivity reaction). Patch tests with different concentrations have shown that concentrations below 0.05% rarely elicit an allergic response in sensitised persons. However, patch-tests using concentrations of 2% or more are often unreliable, as a positive response may be due to a direct irritant response (IPCS 1989, IARC 1995). IPCS (1989) states that skin sensitisation do only occur at exposure to formaldehyde solutions with a higher concentration than 2% (no reference given in the text), and sensitisation from cosmetic products e.g. shampoos is considered unusual.
A Nordic expert group found that data from human experiments suggested that the threshold for sensitisation induced by formaldehyde would lie in a formaldehyde concentration range of 0.037-0.37%. This was the result from a study in which persons were induced with ten epicutaneous applications of different concentrations of formaldehyde (Draize procedure). At 0.037% none of 45 test persons developed sensitisation, whereas at 0.37% 4 out of 85 persons (4.5%) were sensitised (Marzulli & Maibach 1974). In a human maximisation study 72% of the test persons induced with 1.85% formaldehyde solution were sensitised (Nord 1991).
In a series of patch tests the lowest formaldehyde concentration for provocation of allergic response in sensitised persons was found to a formaldehyde content of 150 ppm, and the no effect concentration was found to 80 ppm. In another study where formaldehyde were applied in the axilla a 30 ppm solution elicited positive response in four of nine sensitised persons (Nord 1991).
In a recent study, Flyvholm et al. (1997) found a 250 ppm formaldehyde solution under occlusive dressing as the lowest concentration that caused positive response in a provocation test with formaldehyde sensitised persons. No persons responded at 50 ppm. Using non-occluded exposure no reaction was found at the highest concentration tested at 10000 ppm.
3.2 Long term toxicity
Occupational inhalation exposure
The possibility that formaldehyde may induce pathological or cytogenetic changes in the nasal mucosa has been examined in several occupational studies. In these studies from the wood, furniture and resin industry the workers were exposed to formaldehyde at average levels up to 2.4 mg/m3. Examination of nasal biopsies from the workers more frequently revealed signs of inflammation and histological changes (hyperplasia, metaplasia and dysplasia) compared to non-exposed groups. However, the findings were not always statistically significant and no firm relation between the adverse findings and exposure level or duration could be found. (IARC 1995).
Also pulmonary function has been assessed in several studies with formaldehyde exposed workers. The formaldehyde levels were <0.02 - >6.0 mg/m3 and in most of the studies formaldehyde alone or in combination with other agents caused transient, reversible declines in lung function, but with no evidence of a chronic decrement in lung function (IARC 1995).
In a recent evaluation of an expert panel it was concluded based on the epidemiological data from the occupational environment that no long term effects such as emphysema or chronic obstructive pulmonary disease could be found from occupational data with formaldehyde exposure in the range 0.4-2.0 ppm, while at higher levels (above 2 ppm) reductions in pulmonary function have been recorded (Paustenbach et al. 1997).
3.3 Reproductive / developmental effects
No conclusive study results are reported regarding formaldehyde and toxicity to reproductive system or to developing foetuses in humans (IARC 1995, IPCS 1989).
3.4 Genotoxic effects
A series of studies on cytogenetic effects (chromosomal aberrations, sister chromatid exchange in peripheral lymphocytes) of formaldehyde in humans has been published. Both positive and negative results were obtained, but interpretation of the data was found to be difficult because of small number of subjects studied and inconsistencies in the findings. (IARC 1995).
A significant excess of micronucleated cells in nasal scrapes from the mucosa was found in formaldehyde exposed workers from the wood industry. However, no dose-response relation could be found and mixed exposure to wood dust occurred. (IARC 1995).
3.5 Carcinogenic effects
More than 30 epidemiological studies have been performed for determining the relationship between exposure to formaldehyde and cancer in humans. From two recent meta-analyses of these studies a causal relationship was suggested for occupational exposure to formaldehyde and elevated risk for the development of sinonasal and nasopharyngeal cancers (Blair et al. 1990, Partanen et al. 1993).
On the overall human data the International Agency for Research on Cancer (IARC 1995) concluded that there was only limited evidence for carcinogenic effects of formaldehyde in humans. nevertheless, the suggested relationship between formaldehyde exposure and cancer of the nasopharynx and the nasal cavities was noticed. It was found that industrial cohorts showed low or no increased risk for lymphatic or haematopoietic cancer, and cohort studies of embalmers and anatomists tended to show excess risks for cancers of the brain, however, based on small numbers. Based on the overall human and animal data IARC placed formaldehyde in group 2A "probably carcinogenic to humans".
More recent epidemiological data adds further support to a causal relationship between formaldehyde exposure and nasal cancer. Hansen & Olsen (1995) identified 265 Danish companies in which formaldehyde was used. From the Danish Cancer Register 3304 cancer patients having worked more than 10 years in these companies were registered (2041 men and 1263 women). Among these cancer patients the only increased cancer risk was found for nasal cancer (RR for men: 2.3 (1.3-4.0, 95% confidence limit); RR for women: 2.4 (0.6-6.0, 95% confidence limit). In a subgroup of blue-collar workers with no probable wood-dust exposure (a major confounder for nasal cancer) the relative risk for nasal cancer was 3.0 (1.4-5.7).
4. Toxicity, animal data
4.1 Short term toxicity
Inhalation
The LC50-value for a 4-hour exposure is reported to 578 mg/m3 for rats and 497 mg/m3 for mice (IPCS 1989).
Formaldehyde is a potent respiratory tract irritant as exposure to 3.8 mg/m3 produces a 50% decrease in respiratory frequency (= RD50) in mice. At this concentration mild histopathological lesions in the anterior nasal cavity was found in the animals following six hours exposure each day for five days (IARC 1995).
Short term, repeated exposures (7-25 mg/m3) of rats produced histological changes in the nasal epithelium, such as degeneration, inflammation, necrosis, squamous metaplasia, and increased cell proliferation (IPCS 1989). In rats exposure to 2.5 mg/m3 formaldehyde 6 hours/day for three days has resulted in increased cell proliferation in the nasal and tracheal mucosa (Roemer et al. 1993).
There is growing evidence that it is the concentration rather than the cumulative dose that determines cytotoxic effects of formaldehyde on the nasal mucosa of rats; concentrations below 1 mg/m3 do not lead to cell damage and hyperplasia (IPCS 1989).
At 0.6 mg/m3 irritation of eyes, nose, and throat were observed in experimental animals (IPCS 1989).
Oral administration
The LD50-value for a single oral administration is reported to 800 mg/kg in rats and 269 mg/kg in guinea pigs (IPCS 1989).
Dermal contact
The LD50-value for a single dermal application is reported to 270 mg/kg for rabbits (IPCS 1989).
Skin irritation
Application of 0.1-20% formaldehyde solutions on the skin of rabbit and guinea pig resulted in mild to moderate irritation (IPCS 1989).
Eye irritation
Experimental application of a drop formalin (approx. 35% formaldehyde solution) to rabbit and guinea pig eyes has caused severe reactions, with oedema of the cornea and conjunctiva, and iritis, graded 8 on a scale to 10 at twenty-four hours but with a tendency to recovery in the course of a month or two. A 0.05% solution applied to rabbit eyes caused complete loss of the top layer or corneal epithelial cells. (Grant 1986).
Skin sensitisation
Formaldehyde has been found to be a skin sensitiser in several animal experiments including the guinea pig maximisation tests in epicutaneous maximisation tests, and in epidermal Draize tests. In the most sensitive guinea pig strain sensitisation has been induced with a 0.01% formaldehyde solution (Nord 1991).
In inhalation experiments guinea pigs exposed to formaldehyde vapours caused skin sensitisation, without causing pulmonary hyperreactivity (IPCS 1989).
4.2 Long term toxicity
Inhalation
A range of subchronic and chronic inhalation experiments have been performed in different animals where especially the early effects in the nasal mucosa have been determined, as these effects may be related to formation of the nasal tumours that occur at longer exposure periods.
From these studies no increases in cell turnover or DNA synthesis have been found in the nasal mucosa after subchronic or chronic exposure to concentrations £ 2 ppm (2.4 mg/m3), while small site-specific increases in the rate of cell turnover and increased DNA synthesis were observed in rats at 3 ppm (3.7 mg/m3) and 6 ppm (7.3 mg/m3), respectively (IARC 1995).
The subchronic and chronic studies have demonstrated that the occurrence and the severity of lesions in the nasal mucosa are more linked to the concentration of formaldehyde than to the total dose or exposure duration (IARC 1995).
Oral administration
Formaldehyde administered in drinking water to rats for a 2 year period has resulted in lesions of the forestomach and the glandular stomach of the animals (raised limiting ridge of the forestomach and gastritis and hyperplasia of the glandular stomach) (IARC 1995).
Til et al. (1989) exposed rats to formaldehyde in drinking water for up to 24 months. The formaldehyde content was continuously adjusted so that the rats would receive daily doses of 5, 25 and 125 mg/kg/day (formaldehyde concentration in drinking water: 0.002, 0.026, and 0.19%). However, the actual daily doses for male/female rats were: 0/0, 1.2/1.8, 15/21 and 82/109 mg/kg per day. At the highest dose level significant lesions in the stomach and forestomach were seen, while no differences compared to control animals were noted at the second highest dose level. Furthermore, significant increased incidences of papillary necrosis of the kidneys were observed at the highest dose level in both male and female animals. A NOAEL of 15 mg/kg per day for male and 21 mg/kg per day for female rats (formaldehyde concentration of 0.026%) was concluded by the authors.
In a study by Tobe et al. (1989) where rats were administered drinking water with a formaldehyde content of 0, 0.02, 0.10, and 0.50% a LOAEL for lesions of the forestomach and glandular stomach was 0.1% (corresponding to 50 mg/kg/day) and NOAEL was found to 0.02% formaldehyde (corresponding to 10 mg/kg/day).
4.3 Reproductive / developmental effects
Formaldehyde administered by inhalation, ingestion or by dermal contact to various rodent species did not in a series of reproductive and developmental toxicity studies exert adverse effects on reproductive parameters or foetal development (IPCS 1989, IARC 1995).
4.4 Genotoxic effects
Formaldehyde has been reported positive in in vivo tests in rats, where chromosomal anomalies have been found in lung cells after inhalation, while micronuclei formation in the gastrointestinal tract was found after gavage administration. Inhalation of formaldehyde leads to DNA-protein cross-links in the nasal mucosa of rats and monkeys. (IARC 1995).
In in vitro tests formaldehyde induced mutation, gene conversion, DNA strand breaks, and DNA-protein cross links in fungi, and mutation and DNA damage in bacteria. Further, formaldehyde induced DNA-protein cross-links, DNA single-strand breaks, chromosomal aberrations, sister chromatid exchange and gene mutation in human cells in vitro. (IARC 1995).
In general, the available data show that formaldehyde is genotoxic, especially when high concentrations act directly on cells (gene and chromosome mutations). Addition of metabolising systems tends to reduce the activity of formaldehyde. (IPCS 1989).
4.5 Carcinogenic effects
Inhalation
Inhalation carcinogenicity studies have been performed with mice, various strains of rats, and hamsters.
hamsters
In two studies with hamsters one with exposure to 10 ppm (12.3 mg/m3) formaldehyde 5 hours/day, 5 days/week for lifetime and one with exposure to 30 ppm (36.9 mg/m3) 5 hours/week for lifetime no tumours of the nasal cavities were found (IARC 1995).
mice
B6C3F1 mice (120 animals of each sex in each exposure group) were exposed to 2, 5.6, and 14.3 ppm formaldehyde (2.5, 6.9, 17.6 mg/m3) 6 hours/day, 5 days/week for up to 24 months. After 24 months of exposure two of 17 remaining animals (reduced number of animals due to interim sacrifices) in the high dose group had developed squamous-cell carcinomas, whereas no tumours were found at the other dose levels. At 14.3 ppm a variety of non-neoplastic lesions in the nasal cavities were commonly found. (IARC 1995).
rats
Fischer rats (120 animals of each sex per dose groups) were exposed to formaldehyde at 2, 5.6, 14.3 ppm (2.5, 6.9, 17.6 mg/m3) 6 hours/ day, 5 days/week for up to 24 months (Kerns et al. 1983, IARC 1995).
The histopathological findings with respect to neoplastic lesions are given in Table 4.1.
Table 4.1 Neoplastic lesions in the nasal cavities of Fischer 344 rats exposed to formaldehyde vapour (from IARC 1995).
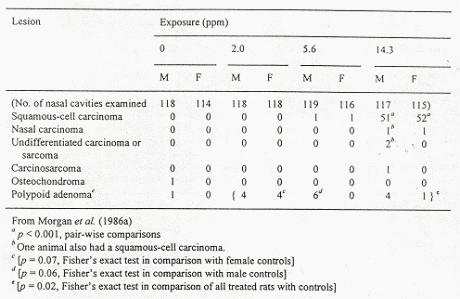
Other histopathologic findings from the study were:
2.0 ppm: after 12 months of exposure purulent rhinitis, epithelial dysplasia and squamous metaplasia in the anterior part of the nasal turbinates were observed. Three months post-exposure (month 27) there was a significant decrease in the frequency of metaplasia.
5.6 ppm and 14.3 ppm: exposure related increase in intensity of the effects observed at 2.0 ppm. The lesions were observed also in the deeper parts of the nasal cavities (Kerns et al. 1983).
Monticello et al. (1996) exposed groups of 90-147 male F344 rats to 0, 0.7, 2, 6, 10, and 15 ppm of formaldehyde (0.9, 2.5, 7.4, 12, and 18 mg/m3) 6 hours/day, 5 days/week for up to 24 months. Six rats in each groups were interim killed at 3, 6, 12 and 18 months. In the 6, 10 and 15 ppm groups squamous cell carcinoma in the nasal cavities were found in 1/90, 20/90, and 69/147 of the animals, respectively, while no nasal tumours occurred at the lower exposure levels.
In the study, cell proliferation in different parts of the nasal mucosa was determined using a DNA labelling technique. From these data significant increases in cell proliferation occurred only at the two highest exposure levels indicating a threshold at about 6 ppm for the induction of cell proliferation. Furthermore, the occurrence of tumours in different part of the nasal mucosa was correlated to the degree of increased labelling index for the cell populations in the specific areas. The authors suggested that the cell proliferation induced by high formaldehyde exposure would make the tissue more vulnerable for the genotoxic effects of formaldehyde.
In another study, 100 Sprague Dawley rats were exposed to 14.2 ppm formaldehyde (17.5 mg/m3) 6 hours/day, 5 days/week during lifetime. At the end of the experiment 38 squamous-cell carcinomas of the nasal cavities and 10 polyps or papillomas were observed compared to no neoplastic changes in a group of 99 control animals (Sellakumar et al. 1985, IARC 1995).
In another study nine groups of 43-45 Wistar rats were exposed to 10 ppm (12.3 mg/m3) and 20 ppm (25 mg/m3) formaldehyde 6 hours/day, 5 days/week for 4, 8 and 13 weeks. The animals were observed for 117-126 weeks after exposure. The authors concluded that nasal tumours were only induced to a significant extent in the 20 ppm groups, where 6 exposure related cases of nasal tumours occurred among a total of 132 animals. Thus, after 4,8 and 13 weeks exposure 1, 1 and 4 nasal tumours in the 20 ppm groups were found. (IARC 1995).
Oral administration
Three long term oral carcinogenicity studies have been performed with rats:
Groups of 50 male and 50 female Sprague Dawley rats were administered formaldehyde in drinking water at concentration levels of 10, 50, 100, 500, 1000, and 1500 ppm (mg/l) for 104 weeks (Soffritti et al. 1989). [Corresponding to 1, 5, 10, 50, 100, 150 mg/kg b.w. per day according to WHO (1996)]. Two control groups received either pure drinking water or drinking water containing 15 ppm of methanol (the formaldehyde contained 0.3% of methanol as stabiliser).
In the treated groups, dose related increased incidences of leukaemia (leukaemia included observed cases of: lymphoblastic leukaemia and lymphosarcomas plus immunoblastic lymphosarcomas plus other haemolymphoreticular neoplasia) was observed at rates of 3%, 9%, 9%, 12%, 13%, 18% in the dosed groups versus 3.5% in the water control group and 8% in the water/ methanol group.
(Due to differences in the occurrence of spontaneous leukaemia between sexes, it seems relevant to separate the cases on the individual sexes. Furthermore, the cases consisting of "other haemolymphoreticular neoplasia" is not described in more details in the paper and should therefore not without further explanation be added to the cases of lymphoblastic leukaemia and lymphosarcomas and the cases of immunoblastic lymphosarcomas. Taking these consideration into account significant increased incidences of leukaemia still persisted in the two highest dose groups of females and in the highest dose group of males).
Further, increased incidences of benign and malignant neoplasia in the gastro-intestinal tract were observed, especially at the highest dose level (at 1500 ppm: 3/100 having adenoma + adenocarcinoma and 5/100 having leiomyomas + leiomyosarcomas, compared to 0% in each of the two control groups consisting of a total of 300 animals. Further, the rare occurrence of these type of tumours in historical controls were emphasised).
In a two-year study with dosing of drinking water containing 2500 ppm formaldehyde to rats and their offspring only the parent rats showed increased incidence of leukaemia (11% compared to 2.5% in the controls), whereas 5.6% of the parent rats and 17.8% of the offspring developed gastrointestinal tract neoplasia compared to 0% in comparable control groups (Soffriti et al. 1989).
In contrast two other studies showed no evidence of formaldehyde induced carcinogenicity:
Til et al. (1989) exposed groups of 70 male and 70 female Wistar rats to formaldehyde in drinking water. The males/females received doses of 0/0, 1.2/1.8, 15/21, 82/109 mg/kg b.w. per day for up to 24 months (formaldehyde content in drinking water of 0, 20, 260, and 1900 mg/l). Treatment related hyperplastic lesions, ulceration and atrophy were found in the forestomach and glandular stomach. No increased mortality occurred and the incidences of tumours did not differ significantly between the groups. (Til et al. 1989, IARC 1995).
In the third study groups of only 20 Wistar rats of each sex were exposed to drinking water containing 0, 200, 1000, and 5000 ppm for 24 months. Non-neoplastic lesions consisting of squamous- and base-cell hyperplasia, erosion and ulceration were seen in the forestomach and stomachs at the highest dose level. There were no significant differences in the incidence of tumours among the groups. The sensitivity of this study, however, was significantly impaired because of early death in the highest dose group, as only 50% of the animals survived one year and none of the animals survived the 2 year dosing period (Tobe 1989, IARC 1995).
4.6 Combination effects
Irritation
In a study where respiratory tract irritation were correlated to decrease in respiratory frequency in rats, it was found that combination exposure to the three aldehydes formaldehyde, acetaldehyde and acrolein resulted in additive response if the response was fitted to a model of competitive receptor binding kinetics (Cassee et al. 1995).
In another study where combination exposure to the same three aldehydes was performed the lesions in the nasal mucosa of rats were determined. At the lowest levels (formaldehyde 1.2 mg/m3; acetaldehyde 1350 mg/m3; acrolein 0.6 mg/m3, which were levels near the individual NOAELs) no additive effects were found, however, at higher levels (formaldehyde 3.9 mg/m3; acetaldehyde 2700 mg/m3; acrolein 1.5 mg/m3) the combined exposure resulted in more than additive effects (Feron et al. 1995).
Carcinogenicity
Combined exposure has been conducted with formaldehyde plus hydrochloric acid and formaldehyde plus wood dust in experimental animal studies (IARC 1995). In both studies only minor differences in effects and responses were found between the combination exposure group and the formaldehyde exposure group. Thus, no clear conclusion can be drawn with respect to the nature of possible combination effects from these studies.
Tumour promoting effect of formaldehyde has been demonstrated in both mice and rats. Thus oral exposure to both N-nitrosodimethylamine (carcinogenic initiator) and formaldehyde resulted in significantly increased tumourigenic response in mice (tumours in liver, kidney and lung) and rats (papillomas of the forestomach) compared to treatment with either of the substances alone. (IARC 1995).
In an experiments with a single dermal exposure to dimethylbenz(a)anthracene in mice followed by dermal exposures to formaldehyde, the application of formaldehyde was shown to enhance the rate at which skin tumours occurred (IARC 1995).
5. Regulations, limit values
Ambient air
Denmark (C-value): 0.02 mg/m3 (MST 1996).
Drinking water
Denmark: -
WHO (1996): 900 mg/l, based on a 20% allocation of a
TDI value of 150 mg/kg per day. The TDI value is based
on a NOAEL of 15 mg/kg per day from the chronic oral study by Til (1989) using a
uncertainty factor of 100.
Soil
-
OELs
Denmark: 0.4 mg/m3 (At 1996).
Classification
Formaldehyde is classified for acute toxic effects (T;R23/24/25 - toxic by inhalation,
in contact with skin and if swallowed), for corrosive properties (C;R34 - causes burns),
for carcinogenic effects (Carc3;R40 - possible risks of irreversible effects) and for
sensitisation (R43 - may cause sensitisation by skin contact) (MM 1996).
IARC/WHO
Group 2A, based on limited evidence for carcinogenicity in humans and sufficient
evidence from inhalational animal experiments (IARC 1995).
US-EPA
U.S. EPA (1986): 0.08 mg/m3 as 10-6 lifetime risk (calculated from the data by Kerns et al. (1983) using the linearised multistage model) (U.S. EPA 1986 - quoted from IRIS 1992).
U.S. EPA (1991): 3 mg/m3 as 10-6 lifetime risk (using DNA-protein cross-link dosimetry in the nasal cavity as indicator for dose at the target tissue, and considering differences in anatomy of the nasal cavities between rats and primates (resulting in greater retention of formaldehyde in the nasal cavities of rats compared to monkeys)) (U.S. EPA 1991, Hernandez et al. 1994).
5.1 Comments concerning risk assessment
The findings of nasal tumours in rats in specific parts of the nasal mucosa has led to extensive research for determining the underlying causes for the development of these tumours.
Several specific points have been observed (Heck et al. 1990):
tumour dose-response
Studies in rats indicate that the development of squamous cell carcinomas in the nasal cavities follows a very steep dose response relationship, where the development of the tumours starts at 5-6 ppm (with an incidence of about 1%) and rapidly increases in the exposure range up to 14-15 ppm (to an incidence of 44%).
cytotoxicity
No tumours have been seen at exposure levels below cytotoxic levels. A critical concentration (> 2ppm) rather than the total dose determines whether cytotoxicity and lesions in the mucosa occur.
cell proliferation
Cell damage and increased cell proliferation seems to be a prerequisite for the development of tumours in the nasal cavities of the rat.
distribution of dose
In monkeys formaldehyde-induced lesions in the respiratory tract were found to be very similar in nature to those observed in rats. However, the lesions in the respiratory tract in monkeys were more widespread. After single exposure to formaldehyde the concentration of DNA-protein cross-links in the target tissue of the turbinates and anterior nose is significantly lower in monkeys compared to rats (see tissue dosimetry below).
defence mechanisms
Two saturable defence mechanisms have till now been identified in rats: covalent binding of formaldehyde to protein molecules present in the nasal mucus and metabolism by nasal mucosal formaldehydedehydrogenase. Other defence mechanisms such as repair of DNA-protein cross-links, may also be saturable as concentration increases.
tissue dosimetry, DNA-protein cross links
Inhalation of formaldehyde leads to the formation of DNA-protein cross links in the nasal respiratory mucosa. Species differences in the formation of DNA-protein cross links is illustrated in figure 5.1 which shows the relation between 6 hours formaldehyde exposure level in the range of 0.9-7.3 mg/m3 and cross-link formation in rats and rhesus monkeys. In rats the formation of DNA-protein cross-links was non-linear related to the formaldehyde concentration with a more steep dose-response relationship above 3-4 mg/m3 formaldehyde (IARC 1995).
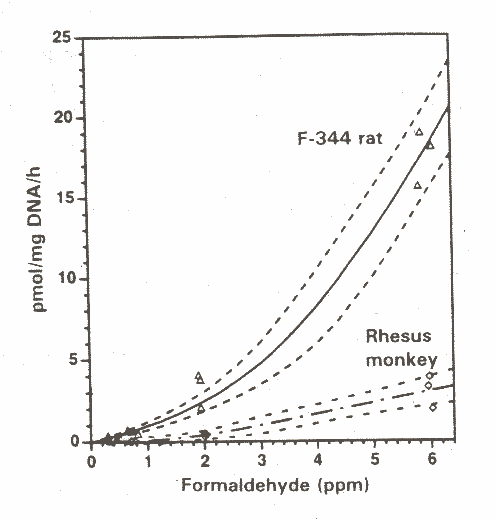
Figure 5.1 Concentration of DNA-protein cross-links formed per unit time in the turbinates and lateral wall/septum of Fischer 344 rats and rhesus monkeys in relation to airborne formaldehyde concentration
(from IARC 1995).
These findings illustrate a more intense exposure of specific part of the nasal mucosa in rats compared to monkeys at identical exposure level.
A pharmacokinetic model to predict the degree of DNA-protein cross-links in humans showed lower formation of DNA-protein cross-links in humans compared to rats and rhesus monkeys. Furthermore, the studies showed that repeated daily exposure did not lead to higher levels of DNA-protein cross-links than single exposure, indicating that no accumulation takes place and that the cross-links in the nasal mucosa is rapidly repaired (IARC 1995).
revision of risk assessment
Due to the large differences of the anatomy of the nasal cavities between rats and primates and due to the shown differences in the formation of DNA-protein cross-links in the nasal target tissue, U.S. EPA re-evaluated their prior risk assessment from 1986. Calculating the cancer risk for monkeys, using DNA-protein cross links as indicator for the target tissue exposure, a risk estimate of 3 x 10-5 was calculated for monkeys at an average exposure level of 0.1 mg/m3 formaldehyde (this corresponds to 3.3 mg/m3 as a 10-6 life-time risk), see Table. 5.1.
Table 5.1 Comparison of upper bound estimates (the upper 95% confidence limits) of human lifetime carcinogenic risk for lifetime exposure to formaldehyde. The estimates are calculated by the use of the linearised multistage model.
| Exposure level | 1987 risk estimates rat dataa | 1991 risk estimates rat datab | 1991 risk estimates monkey-datab |
| 0.1 ppm | 2 x 10-3 | 3 x 10-4 | 3 x 10-5 |
| 0.5 ppm | 8 x 10-3 | 3 x 10-3 | 2 x 10-4 |
| 1.0 ppm | 2 x 10-2 | 1 x 10-2 | 7 x 10-4 |
a: estimated using 1987 unit risk 1.6 x 10-2/ ppm.
b: incorporated rat and monkey dosimetry data.
Comparing the 0.1 mg/m3 risk level a 66 times reduced risk estimate
was obtained by the new approach, where a factor 2.5 could be ascribed to a new linearised multistage model and a factor 25 to DNA-protein cross link dosimetry and the differences between rat and monkey. (U.S.EPA 1991, Casanova et al. 1991, Hernandez et al. 1994, Conolly et al. 1995).
6. Summary
Description
Formaldehyde is a colourless, flammable gas, highly soluble in water. The most common commercially available form is a 30-50% aqueous solution.
Industrial use
The main use of formaldehyde is in the production of resins for various industries e.g. the wood industry. Formaldehyde itself is used for preservation, disinfection and embalming of biological specimens. It is used as a preservative in many consumer products e.g. cosmetics.
In biological organisms
Formaldehyde is an essential metabolic intermediate in the biosynthesis of purines, thymidine and certain amino acids, and is thus expected to occur in most life-forms. Naturally occurring concentration of free and reversible bound formaldehyde in the blood of humans has been determined to 2.6 mg/kg.
In food items formaldehyde occurs at a concentration of 3-60 mg/kg in fruits and vegetables and at levels of about 6-20 mg/kg in fish and meat.
Environment
The background level for formaldehyde in air is <1mg/m3, but in urban areas where formaldehyde is a part of vehicle exhaust and also generated from photochemical oxidation of volatile hydrocarbons levels of 1-20 mg/m3 have been reported.
Rainwater is reported to contain 0.1-0.2 mg formaldehyde/ kg.
Drinking water in general contains less than 0.1 mg/l.
Human exposure
Indoor air levels of 10 to 1000 mg/m3 are reported due to vaporising from furniture and building materials. Tobacco smoke may significantly contribute to the formaldehyde air level. Thus the indoor climate may result in a daily exposure of several mg of formaldehyde.
The daily intake from food is estimated to 1.5-14 mg/day.
The dermal use of formaldehyde containing cosmetics is not considered to contribute to systemic exposure of formaldehyde as absorption is regarded to be negligible.
Toxicokinetics
When inhaled the highly hydrophilic formaldehyde is retained by the mucosa of the upper respiratory tract. The reactive formaldehyde is thought to react with proteins in the mucous layer or with macromolecules of the epithelial cells. Thus, no increase in formaldehyde blood levels could be determined in rats and monkeys exposed to either 17.6 or 7.3 mg/m3.
After oral exposure formaldehyde is rapidly absorbed and may be either metabolised and eliminated as CO2 or be incorporated into purines and amino acids via the one-carbon pool biosynthesis.
No data indicate increased levels of formaldehyde in blood following formaldehyde exposure by the inhalational, oral or dermal route. However, exposure to radiolabelled formaldehyde show distribution and metabolic incorporation in tissue and elimination of radioactivity through expired air and urine. These data indicate that formaldehyde almost instantaneously undergoes metabolic conversion to formate (or reacts with macromolecules) before entering the systemic blood stream.
Human toxicity
vapour exposure
Odour, upper respiratory tract irritation and eye irritation are typical effects observed in humans exposed to low levels of formaldehyde. Although a great amount of human data exist with respect to the induction of these effects by formaldehyde exposure, big differences in quality of data and big variability of individual sensitivity makes it difficult to identify a no effect level for sensory irritation. Different expert groups have recently evaluated the data on sensory irritation and the following irritation thresholds were stated:
| Thresholds for sensory irritation, mg/m3 | |
| IPCS (1989) | 0.06-0.12 |
| ACGIH (1991) | 0.1 0.3 (10-20% response level) |
| WHO (1998) | 0.1 (significant irritation starts to occur above this level) |
| Paustenbach et al. (1997) | 0.1 (no effect level for virtually all persons) 0.37 (1-5% response level) |
At higher exposure levels of formaldehyde biting sensation (2.5-3.7 mg/m3) and lachrymation (5-6.2 mg/m3) occur, while pulmonary oedema and danger to life may be the consequence of even higher concentrations (37-60 mg/m3).
Vapour exposure to formaldehyde is generally not considered to produce respiratory tract sensitisation.
From occupational data reduced lung function has been reported in relation to occupational exposure above 2 mg/m3. Workers exposed to formaldehyde more frequently developed inflammation and histological changes in the nasal cavities, and increased incidences of nasal cancer have been found in workers exposed to formaldehyde.
oral exposure
Oral ingestion of few millilitres of concentrated formaldehyde solution (about 40%) may be fatal, resulting in pronounced injury in the oesophagus and the gastrointestinal tract.
skin/ eye contact
Contact with skin with concentrated formaldehyde may cause corrosive burns. Contact with eyes may result in severe injury and loss of vision.
Formaldehyde is a strong skin sensitiser. The lowest concentration resulting in skin sensitisation in human experiments is 0.37% formaldehyde. The lowest concentration for provocation of an allergic response in sensitised persons is 30 ppm (30 mg/kg) for occlusion exposure in the axilla.
Animal toxicity
inhalation exposure
Long term inhalational exposure to rats and mice has resulted in the development of squamous cell carcinomas of the nasal cavities. Especially rats are sensitive showing a steep dose-response relationship for the development of tumours in the range of 7-17 mg/m3.
The development of tumours has been found to be related to delivered target site dose (measured as formaldehyde induced DNA-protein cross links) and tumours were always found to occur at cytotoxic exposure levels, i.e. at levels resulting in cell damage and increased cell proliferation.
Compared to other primates (monkeys) the target tissue in the nasal mucosa of the rats were found to be more heavily exposed. This was found to be related to the structure of the nasal cavities of the rodent, where the more complex anatomy and narrow airflow increases the absorption/ retention of formaldehyde from the inhaled air.
oral exposure
In two long term studies in which rats were dosed with formaldehyde through the drinking water treatment related hyperplastic lesions, ulceration and atrophy developed in the forestomach and glandular stomach at dose levels of 50 and 82 mg/kg/day (drinking water contents of 0.1% and 0.19%), respectively. The corresponding NOAEL were 10 and 15 mg/kg/day (formaldehyde drinking water contents of 0.02 and 0.026%), respectively. No increases in the incidences of tumours were found in these two studies. In one of the studies, however, the mortality was greatly increased at the highest dose level of 0.5% formaldehyde in drinking water, and no animals survived the hole exposure period of 2 years.
In another study using formaldehyde contents of 10, 50, 100, 500, 1000, 1500 ppm in drinking water, a dose related increase in leukaemia was observed among the rats. When cases of leukaemia were separated on sexes and grouping of different types of leukaemia was considered significant increased incidences occurred at the two highest levels. Further, increased incidences of different types of tumours in the gastrointestinal tract were found. No clear dose response was found a the lower levels, however, at 1500 ppm (and in a subsequent study at 2500 ppm) clearly increased incidences occurred. Some of these tumours were noticed to occur spontaneously very rarely.
Reproductive and developmental effects
Formaldehyde did in various experimental studies not show any adverse effect with respect to reproductive parameters and foetal developmental, at dose levels not resulting maternal toxicity.
Genotoxicity
Formaldehyde is considered as a genotoxic substance. In vivo studies have resulted in DNA-protein cross link in the nasal mucosa of rodents and monkeys, in chromosomal anomalies in lung cells of rats and in micronuclei formation in the gastrointestinal tract mucosa. Positive results were obtained in in vitro studies with respect to DNA damage and mutation in bacteria, and DNA-single strand breaks, chromosomal aberrations, sister chromatid exchange and gene mutation in human cells.
7. Evaluation
Inhalation exposure
For inhalation exposure sensory irritation of eyes and respiratory tract should be considered as critical effects from short term exposure. Based on the recent evaluation by WHO (1998) significant irritation in the general population occurs above levels of 0.1 mg/m3, and thus this level may be considered as a NOAEL for the general population. Thus this value will be used as the basis for the limit value in air.
With respect to long term exposure the development of nasal cancer should be considered as critical effect. From experimental animal data there seems to be a threshold at about 5-6 mg/m3 for the development of tumours in the nasal cavities, as none of these tumours were found below this level at which clear cytotoxic effects occur. Several data indicate that cytotoxicity is a prerequisite for the development of cancer.
Some important aspects with respect to cytotoxic effects of formaldehyde should be mentioned. Cytotoxicity in the nasal cavities after long term exposure has been observed down to 2 ppm (2.5 mg/m3) but not at 0.7 ppm (0.86 mg/m3). At dose levels in the 0.1-1 ppm range primates are expected to be about a factor 10 (see table 5.1) less susceptible compared to rats because rats are subjected to more concentrated exposure than primates due to a more narrow and complex anatomy of the nasal cavity resulting in enhanced retention of formaldehyde in certain area of the nasal cavity. Cytotoxicity has been found more related to formaldehyde concentration than the total exposure expressed as the product of concentration and duration (c x t).
The well documented genotoxic potential of formaldehyde argues against a threshold for the carcinogenic effects, as the genotoxic effects are thought to be linked to the carcinogenic effect of the substance. Opponents for a threshold of formaldehyde argue, that the substance due to high reactivity at low levels will react with proteins and macromolecules in the mucous and at the outer cell surface or be metabolised by nasal mucosal formaldehydedehydrogenase which protects against the genotoxic potential of the substance. Thus formaldehyde exposure has to exceed these saturable defence mechanisms before the substance can exert genotoxic activity on a cellular level. Therefore the genotoxic potential may first (or especially) be expressed at higher levels where also cytotoxicity occur, and where increased cell turnover make expression of the genotoxic effects more probable. Such a threshold phenomenon would explain the very steep dose-response relationship for the development of nasal tumours and therefore it seems most relevant to use a threshold approach when making a risk assessment for formaldehyde.
oral exposure
For oral exposure the induction of lesions in the gastrointestinal mucosa, the development of gastrointestinal tumours, the development of histopathological changes in the kidneys, and leukaemia should all be considered as critical effects.
With respect to development of lesions of the gastric mucosa and histopathological changes in the kidneys a NOAEL of 15 mg/kg/day was obtained in a long term study with oral administration of drinking water containing formaldehyde.
One oral study with rats resulted in dose related increase in incidences of leukaemia when formaldehyde was administered in drinking water (from 50 to 1500 ppm). When tumour type and occurrence on sex was considered level of significance was reached at the two highest dose levels of 1000 and 1500 ppm (corresponding to 100 and 150 mg/kg b.w./day) i.e. NOAEL at 500 ppm (corresponding to 50 mg/kg b.w./day). Further increased incidences of different types of gastro-intestinal tumours occurred, most clearly at 1500 ppm (and at 2500 ppm in a subsequent study with only one dose group included).
Thus, effects in the gastro-intestinal tract seem to be the most consistent effects observed in these three oral long term studies. As for effects in the respiratory tract, it can not be excluded that the irritant effects of formaldehyde could give rise to some of the tumour types observed in the gastro-intestinal tract. Thus, the NOAEL value of 15 mg/kg b.w./day will be used as basis for the calculation of a TDI value.
dermal exposure
The induction of dermal sensitisation and the development of allergic response are considered as critical effects from dermal formaldehyde exposure. The LOAEL/NOAEL values for induction of dermal sensitisation has been found at formaldehyde concentrations of 0.37% / 0.037% formaldehyde after ten times 48-72 hours of occlusive exposure to formaldehyde. The LOAEL for provocation of allergic responses in formaldehyde sensitised persons was found to exposure of a 30 ppm formaldehyde preparation in the axilla (occlusive dressing for 48 hours). Exposure by occlusive dressing placed on the back resulted in LOAEL at 250 ppm formaldehyde and NOAEL of 50 ppm for the development of allergic response in sensitised persons.
8. TDI, limit values
8.1 TDI
A NOAEL of 15 mg/kg b.w./day is used as the basis for the TDI calculation (see above).
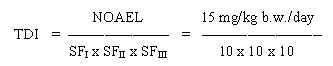
= 0.015 mg/kg b.w./day
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 10 to account for the severity of the effects of formaldehyde (indications of carcinogenic effects at higher dose levels).
Allocation
The general population is predominantly exposed to formaldehyde from food items. Therefore, only 10% of the oral TDI is allocated to ingestion of soil and 10% to drinking water.
8.2 Limit value in soil
Ingestion
Based on the TDI of 0.015 mg/kg b.w. per day and assuming a daily ingestion of 0.2 g soil for a child weighing 10 kg (wchild), a limit value is calculated:
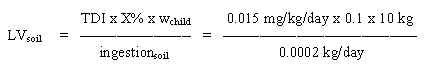
= 75 mg/kg soil
Dermal contact
Dermal contact to soil polluted with formaldehyde may theoretically also be critical for formaldehyde sensitised people. However, a level of 75 mg/kg soil is considered to protect against skin reactions, as a NOAEL of 80 mg/kg has been found in clinical testing with formaldehyde solutions in sensitised people.
8.3 Limit value in drinking water
Based on the TDI of 15 mg/kg b.w. per day and assuming a daily ingestion of 2 litres of drinking water for an adult weighing 70 kg (wadult), a limit value is calculated:
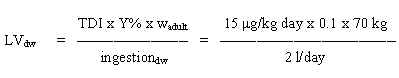
= 53 mg/l
8.4 Limit value in air
A limit value for acute exposure can be calculated based on an overall no effect level for irritation of 0.1 mg/m3 in the general population:

= 0.01 mg/m3
The safety factor SFI is set to 1 as human data is used. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 1, as a NOAEL is used.
A limit value for long term exposure is based on the limit value for acute exposure as mechanistic data from animal experiments strongly suggest that a linkage exists between irritation and carcinogenic effects from long term exposure. Therefore, to account for remaining uncertainties regarding effects from long term exposure and to consider the severity of the effects a limit value of 0.001 mg/m3 as an average exposure level can be calculated. In this calculation SFIII is set to 10.
9. Quality criteria, C-value
9.1 Quality criteria in soil
A limit value of 75 mg/kg soil has been calculated based on toxicological considerations by the oral exposure route. Thus, 75 mg/kg soil is proposed as a quality criterion for formaldehyde in soil.
Furthermore, with respect to evaporation from soil, the C-value should be considered.
Quality criterion
75 mg/kg soil.
9.2 Quality criteria in drinking water
A limit value of 53 mg/l has been calculated based on intake of drinking water. A quality criterion of 50 mg/l is proposed.
Quality criterion
50 mg/l.
9.3 C-value
A limit value of 0.01 mg/m3 has been calculated for short term exposure and a limit value of 0.001 mg/m3 has been calculated for continuous long term exposure.
For formaldehyde a C-value of 0.01 mg/m3 is proposed. Meteorological distribution models have shown that the average exposure level is about 1/40 of the level of the C-value. Therefore a C-value of 0.01 mg/m3 is considered to secure an average exposure level below 0.001 mg/m3, which is the proposed limit value for continuous long term exposure.
Thus formaldehyde is placed in Main Group 1 with a C-value of 0.01 mg/m3.
C-value
0.01 mg/m3, Main Group 1.
10. References
* References not cited in the text.
ACGIH (1991). Formaldehyde. In: TLV's Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 1991-1992. Cincinnati, OH, 664-688.
At (1996). Grænseværdier for stoffer og materialer. Arbejdstilsynets At-anvisning Nr. 3.1.0.2, december 1996.
*ATSDR (1997). Toxicological Profile for formaldehyde. ATSDR, U.S. Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. Draft, 364 p.
Bardana EJ and Montanaro A (1991). Formaldehyde: an analysis of its respiratory, cutaneous and immunologic effects. Annals of Allergy 66, 441-452.
Blair A, Saracci R, Stewart PA, Hayes RB and Shy C (1990). Epidemiological evidence on the relationship between formaldehyde exposure and cancer. Scand J Work Environ Health 16, 381-393.
Casanova M and Heck HA (1991). The impact of DNA-protein cross-linking studies on quantitative risk assessments of formaldehyde. CIIT Activities 11(8), 1-6.
Casanova M, Morgan KT, Steinhagen WH, Everitt JI, Popp JA and Heck HA (1991). Covalent binding of inhaled formaldehyde to DNA in respiratory tract of rhesus monkeys: Pharmacokinetics, rat-to-monkey interspecies scaling, and extrapolation to man. Fundam Appl Toxicol 17, 409-428.
Cassee Fr, Arts JHE, Groten JP and Feron VJ (1996). Sensory irritation to mixtures of formaldehyde, acrolein, and acetaldehyde in rats. Arch Toxicol 70, 329-337.
Cassidy SL, Dix KM and Jenkins T (1983). Evaluation of a testicular sperm head counting technique using rats exposed to dimethoxyethyl phthalate (DMEP), glycerol a-monochlorohydrin (GMCH), epichlorhydrin (ECH), formaldehyde (FA), or methyl methanesulphonate (MMS). Arch Toxicol 53, 71-78.
Chang JCF, Gross EA, Swenberg JA and Barrow CS (1983). Nasal cavity deposition, histopathology, and cell proliferation after single or repeated formaldehyde exposures in B6C3F1 mice and F-344 rats. Toxicol Appl Pharmacol 68, 161-176.
Conolly RB, Andjelkovich DA, Casanova M, Heck HA, Janszen DB, Kimbell JS, Morgan KT and Recio L (1995). Multidisciplinary, iterative examination of the mechanism of formaldehyde carcinogenicity: The basis for better risk assessment. CIIT Activities 15(12), 1-11.
Falk J, Juto J-E and Stridh G (1993). Dose-response study of formaldehyde on nasal mucosa swelling. A study on residents with nasal distress at home. Proceedings from Indoor Air Conference, Helsinki July 4-8, 1993, vol 1, 585-589.
Feron VJ, Groten JP, Jonker D, Cassee FR and Bladeren PJ (1995). Toxicology of chemical mixtures: Challenges for today and the future. Toxicology 105, 415-427.
Flyvholm M-A, Hall BM, Agner T, Tiedemann E, Greenhill P, Vanderveken W, Freeberg FE and Menné T (1997). Threshold for occluded formaldehyde patch test in formaldehyde-sensitive patients. Contact Dermatitis 36, 26-33.
Grammer LC, Harris KE, Cugell DW and Patterson R (1993). Evaluation of a worker with possible formaldehyde-induced asthma. J Allergy Clin Immunol 92, 29-33.
Granby K, Christensen CS and Lohse C (1997). Urban and semirural observations of carboxylic acids and carbonyls. Atm Environ 31, 1403-1415.
Grant MW (1986). Toxicology of the eye, third ed., Charles C Thomas Publisher, Springfield, Illinois, 442-446.
Hansen J and Olsen JH (1996). Erhvervsmæssig formaldehydudsættelse og risiko for cancer. Ugeskrift for Læger 158 (29), 4191-4194.
Heck HA, Casanova M and Starr TB (1990). Formaldehyde toxicity -new understanding. Crit Rev Toxicol 20, 397-426.
Hernandez O, Rhomberg L, Hogan K, Siegel-Scott C, Lai D, Grindstaff G, Henry M and Cotruvo JA (1994). Risk assessment of formaldehyde. J Hazard Mater 39, 161-172.
HSDB (1996). Formaldehyde. In: Hazardous Substances Databank.
IARC (1995). Formaldehyde. In: Wood dust and formaldehyde, IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol 62. International Agency for Research on Cancer, WHO, Lyon. 217-362.
IPCS (1989). Formaldehyde. Environmental Health Criteria. World Health Organisation, International Programme on Chemical Safety, Geneva, 219 p.
IRIS (1997). Formaldehyde. In: Integrated Risk Information System. Database quest, last revision on formaldehyde data: January 1992. US-EPA.
Jordan PW, Sherman WT, King SE, Richmond VA and Raritan NJ (1979). Threshold responses in formaldehyde-sensitive subjects. J Am Acta Dermatol 1, 44-48.
Kerns WD, Pavkov KL, Donofrio DJ, Gralla EJ and Swenberg JA (1983). Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Research 43, 4382-4392.
Marzulli FN and Maibach HI (1974). The use of graded concentration in studying skin sensitisers: Experimental contact sensitisation in man. Fd Cosmet Toxicol 12, 219-227.
MM (1996). The Statutory Order from the Ministry of the Environment no. 829 of November 6, 1997, on the List of Chemical Substances.
Monticello TM, Swenberg JA, Gross EA, Leininger JR, Kimbell JS, Seilkop S, Starr TB, Gibson JE and Morgan KT (1996). Correlation of regional and non-linear formaldehyde-induced nasal cancer with proliferating populations of cells. Cancer Research 56, 1012-1022.
MST (1990). Begrænsning af luftforurening fra virksomheder. Vejledning fra Miljøstyrelsen nr. 6 1990.
Nord (1991). Formaldehyd. Kriteriedokumenter fra et nordisk allergiprojekt. Nord 1991:51, Nordisk Ministerråd, 209-223.
Partanen T (1993). Formaldehyde exposure and respiratory cancer - a meta-analysis of the epidemiological evidence. Scand J Work Environ Health 19, 8-15.
Paustenbach D, Alarie Y, Kulle T, Schachter N, Smith R, Swenberg J, Witschi H and Horowitz SB (1997). A recommended occupational exposure limit for formaldehyde based on irritation. J Toxicol Environ Health 50, 217-263.
Patterson R, Dykewicz MS, Evans R, Grammer LC, Greenberger PA, Harris KE, Lawrence ID, Pruzansky JJ, Roberts M, Shaughnessy MA and Zeiss CR (1989). IgG antibody against formaldehyde human serum proteins: A comparison with other IgG antibodies against inhalant proteins and reactive chemicals. J Allergy Clin Immunol 84, 359-366.
Roemer E, Anton HJ and Kindt R (1993). Cell proliferation in the respiratory tract of the rat after acute inhalation of formaldehyde or acrolein. J Appl Toxicol 13, 103-107.
Schuck EA, Stephens ER, & Middleton JT (1966). Eye irritation response at low concentrations of irritants. Arch Environ Health 13, 570-575.
Sellakumar AR, Snyder CA, Solomon JJ and Albert RE (1985). Carcinogenicity of formaldehyde and hydrogen chloride in rats. Toxicol Appl Pharmacol 81, 401-406.
Til HP, Woutersen RA, Feron VJ, Hollanders VHM and Falke HE (1989). Two-year drinking-water study of formaldehyde in rats. Fd Chem Toxicol 27, 77-87.
Tobe M, Naito K and Kurokawa Y (1989). Chronic toxicity on formaldehyde administered orally to rats. Toxicology 56, 79-86.
U.S.EPA (1991). Formaldehyde risk assessment update. Office of Toxic Substances U.S. Environmental Protection Agency, Washington, DC. Final draft June 11, 1991. Section 5.3: Calculation of risk estimates using DPX 69-77.
Verschueren K (1993). Formaldehyde. In : Handbook of environmental data on organic chemicals 2.ed., Van Nostrand Reinhold Company, New York, 678-683.
WHO (1996). Formaldehyde. In: Guidelines for drinking-water quality. 2nd. edition, Vol. 2. World Health Organisation, Geneva, 837-845.
WHO (1987). Formaldehyde. In: Air Quality Guidelines for Europe. WHO Regional Publications, European Series No. 23, Copenhagen, 91-104.
WHO (1998). Formaldehyde. In: WHO air quality guidelines for Europe, 2nd. Edition. World Health Organisation, Regional Office for Europe, Copenhagen, (being in publication).vol 1 organics. European Centre for Environment and Health.
July 1998 / final
Evaluation of health hazards by exposure to
Glutaraldehyde
and estimation of a limit value in air.
Jens Erik Jelnes
Elsa Nielsen
The Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
Denmark
1. General description
1.1 Identity
| Molecular formula: | C5H8O2 |
| Structural formula: | OHC-CH2-CH2-CH2-CHO |
| Molecular weight: | 100.12 |
| CAS-no.: | 111-30-8 |
| Synonyms: | Glutardialdehyde Glutaral Pentanedial 1,3-Diformylpropane |
1.2 Physical / chemical properties
| Description: | The pure chemical is a colourless oily liquid, but commercial solutions often have an amber tint and an odour similar to spoiled fruit. |
| Purity: | 99.7%. Glutaraldehyde is commonly available as 2, 25, or 50% aqueous solutions with acidic pH. |
| Melting point: | -14 ° C |
| Boiling point: | 187-189 ° C (with decomposition) |
| Density: | 1.061 g/ml |
| Vapour pressure: | 16.5 mmHg (2.2 kPa) at 20°C. There is some confusion about the purity of the test substance used: IUCLID (1996) gives the same value for pure glutaraldehyde and for a 50% aqueous solution, ACGIH (1991) gives a vapour pressure of 0.0152 mmHg (2.0 Pa at 20°C) for a 50% solution. In SUBFAC, vapour pressures (20°C) has been calculated to 24.9 mmHg for a 100% solution, 19.4 mmHg for a 50% solution, and 2.7 mmHg for a 2% solution. |
| Vapour density: | 3.4 (air = 1) |
| Conversion factor: | 1 ppm = 4.2 mg/m3 20° C 1 mg/m3 = 0.240 ppm 1 atm |
| Flash point: | 71 ° C |
| Autoignition temp.: | 225 ° C |
| Solubility: | Water: miscible (aqueous solution: pH 3.7). Soluble in alcohol, benzene, and ether. |
| logPoctanol/water: | - 0.22 |
| Stability: | At acid pH, glutaraldehyde forms an intramolecular ring structure, which
further polymerises. At alkaline pH, glutaraldehyde polymerises into long chain molecules
without ring structures. Glutaraldehyde polymerises when heated. |
| Odour threshold, air: | 0.04 ppm (0.17 mg/m3). |
| Odour threshold, water: | 100 mg/l |
| Taste threshold, water: | 100 mg/l |
| The reliability of the odour and taste thresholds in water could not be evaluated. | |
| References: | Merck Index (1996), IUCLID (1996), Beauchamp (1992). |
1.3 Production and use
Glutaraldehyde is synthesised in a two-step process: ethyl or methyl vinyl ether is reacted with acrolein to produce ethoxy or methoxy dihydropyran, respectively, which is then hydrolysed to produce glutaraldehyde and ethanol or methanol, respectively (CIR 1996).
Next to formaldehyde, glutaraldehyde is the most used aldehyde. It has widespread uses as a bactericide, a tanning agent, a fixative for electron microscopy, as a preservative in cosmetics (maximum 0.1% in EU) and in sterilising surgical, endoscopic, anaesthetic, and dental instruments. In the US, it has many additional uses, e.g. as a direct and indirect food additive, and in packaging materials for food (CIR 1996).
Glutaraldehyde has also been used to treat a number of skin disorders, including epidermolysis bullosa, hyperhydrosis, herpes zoster, herpes simplex, dyshidrosis, onychomycosis, and warts (Beauchamp et al. 1992).
1.4 Environmental occurrence
Glutaraldehyde does not appear to occur naturally.
It may be formed in air by the reaction between cyclic alkenes and ozone and hydroxyl radicals (Beauchamp et al. 1992), however, no concentrations are given.
1.5 Environmental fate
Air
No information has been found.
Water/ waste water
In the DOC die-away-test carried out according to EU guideline, there was a 90-100% biodegradation of glutaraldehyde after 28 days (IUCLID 1996).
The elimination rate of glutaraldehyde in industrial sewage was concentration dependent with 30-60 mg/l being eliminated 100% after 5 hours and 300 mg/l being eliminated < 20% after 7 days (IUCLID 1996).
Bioaccumulation
In a test for bioaccumulation using Escherichia coli and incubation for 30 minutes at pH = 4 and concentrations of 0.1-0.4 mg/l of glutaraldehyde, the bioaccumulation was linear with 0.03 mg/mg (dry weight) at the lower concentration of 0.1 mg/l and 0.13 mg/mg (dry weight) at the concentration of 0.4 mg/l. (IUCLID 1996).
1.6 Human exposure
Humans can be exposed to glutaraldehyde in numerous clinical and occupational settings, as indicated by its many different uses. In Germany, exposure to glutaraldehyde was determined in hospital operating and patients rooms, where cleaning solutions containing glutaraldehyde were used. When a cleaning solution containing 0.025% glutaraldehyde was used, the TLV (0.2 ppm, 0.8 mg/m3) was not exceeded. However, when a cleaning solution containing 0.15% glutaraldehyde was used, 0.57 ppm (2.4 mg/m3) glutaraldehyde was detected in the air. (Binding & Witting 1990 - quoted from Beauchamp et al. 1992).
In two Swedish hospitals, where a 2% alkaline glutaraldehyde solution was used to disinfect equipment, the exposure levels ranged from <0.01 to 0.57 mg/m3, with most of the measurements being in the range of 0.02 to 0.14 mg/m3 (Norbäck 1988).
2. Toxicokinetics
2.1 Absorption, distribution
Inhalation
No information has been found.
Oral intake
No information has been found.
Dermal contact
A flow-through skin penetration chamber has been used to determine the in vitro skin penetration of glutaraldehyde over a 6 hours period of 0.75 and 7.5% [1,5-14C]-glutaraldehyde on excised skin from Fischer 344 rats, CD-1 mice, Hartley guinea pigs, New Zealand white rabbits, and human beings. Total recovery from all species ranged from 75-92% for both concentrations. Overall, <0.5% of the 0.75% and <0.7% of the 7.5% glutaraldehyde solution was absorbed through the skin of animals. For human breast skin in vitro, approximately 0.2% of the applied radioactivity penetrated the skin for both doses tested, and around 5% of the radioactivity was bound to the skin. About 70% of the dose was unabsorbed and total recovery was 75 and 76% for the two doses, respectively. (Frantz et al. 1993).
2.2 Elimination
Metabolism
The metabolism of glutaraldehyde has not been studied in detail. It has been suggested that glutaraldehyde is oxidised to mono- or dicarboxylic acids by aldehyde dehydrogenase and then further oxidised through acidic intermediates to carbondioxide, which is expired. (McKelvey et al. 1992).
Excretion
After an intravenous dose of a 0.075 or a 0.75% 14C-glutaraldehyde solution to rats (0.2 ml) and rabbits (2.5 ml), the excretion of radioactivity was followed. With the 0.075% solution, 75-80% of the dose in the rat and 66-71% in the rabbit was recovered as 14 C-carbon dioxide during the first 24 hours following administration; 80% of this carbon dioxide was recovered during the first 4 hours. With the 0.75% solution, the proportion of 14carbon dioxide decreased and the amount of radioactivity recovered in the urine, tissues and carcass increased. The average plasma concentration increased ten fold with a ten fold increase in concentration but the tissue concentration increased by a greater factor. Analyses of individual organs for radioactivity 24 hours after dosing revealed the highest amount of radioactivity in the lungs followed by blood cells, spleen, kidney, adrenal, thyroid, and lung. (McKelvey et al. 1992).
Groups of four male and four female Fischer 344 rats received a dermal dose of 0.2 ml 0.075%, 0.75% or 7.5% 14C-glutaraldehyde solution on an area of 7x7 cm2 of the clipped dorsal skin. The treated area was covered by an occlusive bandage for 24 hours. Urine, faeces, and carbon dioxide was collected during the dosing period. After the dosing period, a material balance was set up based on the parameters given above and other relevant items, where radioactivity could be found, e.g. cage wash and application rod. Total recovery of radioactivity ranged from 75 to 61%, with the majority of the radioactivity being found at the application site (60-45%). Urinary excretion amounted to 1.71 to 0.54% of the dose, with the lower values being found at the 7.5% dose. Faecal excretion amounted to 0.59 to 1.05% of the dose without any dose relationship but females tended to excrete more radioactivity via faeces. Expired carbon dioxide contained 0.57-3.2% of the dose, with the higher values being found in females and in both sexes dose-dependently increasing in the 7.5% dose group. Tissues and carcass contained 0.20-1.02%, and 0.97-3.08% of the radioactivity, respectively. (McKelvey et al. 1992).
Groups of two male and two female rabbits received a dermal dose of 2.5 ml 0.75% or 7.5% 14C-glutaraldehyde solution on an area of 12x12 cm2 of the clipped dorsal skin. The clipped area was covered by an occlusive bandage for 24 hours. Urine, faeces, and carbon dioxide was collected during the dosing period. After the dosing period, a material balance was set up based on the parameters given above and other relevant items where radioactivity could be found, e.g. cage wash and application rod. Total recovery ranged from 71 to 100% with 45 to 31% of the radioactivity being found at the application site. Urinary excretion amounted to 2.1-12.4% of the dose without any dose relationship. Faecal excretion amounted to 0.45 to 1.1% of the dose. Expired CO2 contained 2.4 to 17.3% of the dose with the higher values being found in the 7.5% dose group. Carcass contained appreciable amounts of radioactivity with 4.7 to 36.2% of the radioactivity being found, apparently higher amounts were found at the low dose, but great variability existed between the individuals. In tissues 0.9 to 1.9% of the radioactivity was found with higher values in the high dose group. (McKelvey et al. 1992).
2.3 Toxicological mechanisms
Many of the uses of glutaraldehyde are related to its ability to react with and cross-link proteins. It can react with the a -amino groups of amino acids, the N-terminal amino groups of peptides and the sulfhydryl group of cysteine. The predominant site of reaction in proteins is the e -amino group of lysine. (Beauchamp et al. 1992).
Little information is available on the reaction of glutaraldehyde with DNA or the components of DNA. Products were formed on reaction of glutaraldehyde with deoxyadenosine, deoxyguanosine and deoxycytidine, but not with deoxythymidine. The adducts formed with deoxyadenosine were unstable but those formed on reaction with deoxyguanosine were relatively stable. (Beauchamp et al. 1992).
3. Human toxicity
3.1 Short term toxicity
Inhalation of glutaraldehyde at vapour levels below 0.8 mg/m3 (0.2 ppm) has been reported to cause nose and throat irritation, nausea and headaches (Burge 1989 - quoted from Beije & Lundberg 1997).
A patient was exposed to residual glutaraldehyde via a crack in an anaesthesia mask, this resulted in eye irritation. No information on exposure level is given. (Murray & Ruddy 1985 - quoted from Beauchamp et al. 1992).
Glutaraldehyde solutions may cause mild to severe irritation in the skin, depending on the concentration of the solution and the duration of exposure/contact (Beije & Lundberg 1997).
3.2 Long term toxicity
Inhalation
In studies at hospitals (NIOSH 1991 - quoted from Beije & Lundberg 1997), a relationship between exposure to glutaraldehyde and irritation of eyes and upper respiratory pathways has been demonstrated. The concentration of glutaraldehyde was 0.2 ppm (0.8 mg/m3) or higher. After reconstruction of the occupational setting, the concentration was lowered to 0.1 ppm (0.4 mg/m3) or less and there were no symptoms of irritation.
Norbäck (1988) carried out a questionnaire study aimed to investigate skin and respiratory symptoms among hospital personnel working in areas with possible glutaraldehyde exposure. The glutaraldehyde solution used was a 2% alkaline solution. To be regarded as an area with possible glutaraldehyde exposure at least one litre of the solution should be used per month in the unit. From the questionnaire, the staff of the units selected were divided into an exposed and an unexposed group. The exposed group consisted of 39 persons and the unexposed group of 68 persons. As unexposed, one handled glutaraldehyde less than once a month. Air sampling revealed exposure levels from <0.01 to 0.57 mg/m3 with most determinations being in the range of 0.02 to 0.14 mg/m3. In the comparison between the exposed and the unexposed group, the exposed group reported nasal catarrh, nasal obstruction, smarting of the throat, headache, nausea, rashes on the hands, and eczema significantly more often than the unexposed group. When the mean number of symptoms recorded was compared with the number of days of exposure to glutaraldehyde within the last 6 months, a clear exposure and symptom relationship was found, with a mean number of 5 symptoms being recorded for the group with more than 20 exposure days.
From 65% of 167 nurses working in endoscopy units there have been complaints of eye irritation, skin irritation, headache and cough or shortness of breath. Where measurements were performed, the air conccentration of glutaraldehyde was less than 0.2 ppm (0.8 mg/m3) (Calder et al. 1992 - quoted from Beije & Lundberg 1997).
Nine members of an endoscopy unit were surveyed to find symptoms associated with exposure to vapour from 2% glutaraldehyde in a disinfectant. Air samples taken for one hour during cold sterilisation ranged from 0.05 to 0.12 ppm (0.2 - 0.5 mg/m3). Eight of the workers reported ocular and nasal irritation. (Jachuck et al. 1989 - quoted from CIR 1996).
Four nurses working in endoscopy units complained of respiratory symptoms when exposed to a disinfectant containing 2% glutaraldehyde. In tests designed to simulate occupational exposure, each nurse had three 20-minutes exposures, separated by 40 minutes intervals. Two had positive reactions, one had both immediate and delayed responses, and one had an isolated delayed response. No information on exposure levels is given. (Corrado et al. 1986).
A surveillance of occupational asthma found that 3 of 8 workers exposed to glutaraldehyde were symptomatic (Matte et al. 1990 - quoted from CIR 1996).
Eight workers were referred for investigation of suspected occupational asthma following direct or indirect exposure to glutaraldehyde at work. Seven of them had peak expiratory flow records suggestive of occupational asthma and positive specific bronchial challenge tests to glutaraldehyde. The mean level of glutaraldehyde during the challenge test was 0.068 mg/m3. Thirty personal air samples were collected in 13 hospital endoscopy units, twelve of these used manual methods of disinfecting. The mean personal short term air concentrations of glutaraldehyde was 0.16 mg/m3. (Gannon et al. 1995).
Dermal contact
In an irritancy test, a 10% solution of glutaraldehyde was applied to the anterior, lateral, and posterior ankle and posterior heel of 12 subjects 5 days/week for 4 weeks, and thereafter 3 days/week for further 4 weeks. No irritation (erythema, pruritus, or isolated vesicles and papules) was observed during the first week, however, in 11 subjects the skin was discoloured after 5 applications. During the second week, all subjects were significantly discoloured and 5 subjects had minimal irritation on the anterior ankle which subsided during the third and fourth weeks. One of the 5 subjects became sensitised to glutaraldehyde. During the last four weeks of application, there was no evidence of irritancy, even among those who had previously experienced some irritation. (Reifenrath et al. 1985 - quoted from Beije & Lundberg 1997).
Glutaraldehyde is a skin sensitizer. There are many case reports and smaller studies on allergic contact dermatitis to glutaraldehyde (CIR 1996), larger studies include the following:
Among 657 patients with eczematous dermatitis patch-tested with 2% glutaraldehyde, one showed positive reaction. (Angelini et al. 1985 - quoted from CIR 1996).
Among 884 patients patch-tested with 0.1% glutaraldehyde, two subjects had allergic reactions, one of which had clinical relevance. (Hansen & Menne 1990 - quoted from CIR 1996).
Among 160 patients patch-tested with 1% glutaraldehyde (pH 7.5), no positive reactions occurred. (Juhlin & Hansson 1968 - quoted from CIR 1996).
Glutaraldehyde was tested for sensitisation in 102 male subjects. Ten occlusive induction patches containing 0.1% glutaraldehyde in petrolatum were applied to the upper lateral portion of the arm for 48 to 72 hours over 3 weeks. A non-treatment period of 2 weeks was followed by an occlusive challenge patch containing 0.5% glutaraldehyde in petrolatum. No sensitisation was observed among the 102 men. The experiment was repeated with 30 men and with 5% glutaraldehyde induction patches and a 0.5% glutaraldehyde challenge patch. Seven (23.3%) of the men were sensitised. (Marzulli & Maibach 1974 - quoted from CIR 1996 and from Beije & Lundberg 1997).
Patch tests were performed on 109 volunteers (males and females) using 0.1, 0.2, and 0.5% aqueous solutions of glutaraldehyde with the same concentration being used for induction and challenge. For induction, the glutaraldehyde doses were applied to the skin of the backs under occlusion for 48 to 72 hours. A total of ten induction applications were made over a 3 week period. Two weeks after removal of the final induction patch, a challenge patch was applied under occlusion for 48 hours to a site not used for induction. The reaction was recorded 24 hours after removal of the challenge patch. The two lowest doses produced no evidence for a sensitisation reaction, but at 0.5% there was a definite reaction to the challenge patch in one of the 109 subjects. While 0.1 and 0.2% glutaraldehyde solutions were not significantly irritating to the skin, a 0.5% solution produced mild to moderate local erythema in 16 of the 109 subjects. (Ballantyne & Berman 1984 - quoted from CIR 1996 and from Beije & Lundberg 1997).
3.3 Reproductive / developmental effects
No data on reproductive effects following exposure to glutaraldehyde have been found in the literature.
The assessment of spontaneous abortions and foetal malformations have been studied in Finnish hospital nurses and staff who had been exposed to glutaraldehyde used as a sterilising agent. No increase in risk of either endpoint was found. (Beije & Lundberg 1997).
3.4 Genotoxic effects
No data on genotoxic effects following exposure to glutaraldehyde have been found in the literature.
3.5 Carcinogenic effects
A mortality study has been performed on 186 male workers assigned to glutaraldehyde production or drumming from 1959 to 1978 and followed through 1988. Exposure data (available from 1977 to 1992) ranged from 0.01 to 0.17 ppm (0.04-0.7 mg/m3) with a TWA (8-hour time-weighted average) of 0.05 ppm (0.2 mg/m3) but suggest somewhat higher levels in 1977 and 1978 (TWA of 0.08 and 0.07 ppm (around 0.3 mg/m3), respectively). Based on comparison to the general US population, there was a statistically significant deficit of deaths due to all causes, with 14 deaths observed among the 186 study subjects and 25.4 expected. The rate for total malignant neoplasms was less than expected, with 4 cancer deaths versus 6.1 expected. The 4 cancers included one each due to stomach, lung, and brain and a death due to lymphosarcoma. (Teta et al. 1995).
4. Toxicity, animal data
4.1 Short term toxicity
Inhalation
LC50-values (4 hours exposure) in rats of 0.1, 0.17, 0.29-0.39, 0.8, 0.48 mg/l have been reported (IUCLID 1996).
In male Fischer 344 rats exposed (72 hours) to 10, 20 or 40 mM glutaraldehyde by intra-nasal instillation, no lesions were observed at the lowest concentration. Following instillation of 20 or 40 mM glutaraldehyde, acute inflammatory changes (neutrophilic infiltrates and epithelial erosion) as well as extensive regions of respiratory epithelial hyperplasia and squamous metaplasia were observed; the effects were dose-related. Increased cell proliferation was also observed in these groups. The lesions observed resembled, both in nature and in severity, the changes observed after acute inhalation exposure of rats to carcinogenic concentrations of formaldehyde gas. (St Clair et al. 1990 - quoted from Beije & Lundberg 1997).
In 2-week inhalation studies, groups of 5 F344/N rats and 5 B6C3F1 mice of each sex were exposed to vapours of glutaraldehyde by whole-body inhalation at concentrations of 0, 0.16, 0.5, 1.6, 5, and 16 ppm (0, 0.7, 2.1, 6.7, 21, 67 mg/m3) for 6 hours per day, 5 days per week. (NTP 1993 - quoted from Beije & Lundberg 1997, CIR 1996, and from Ballantyne 1995).
All rats exposed to 5 or 16 ppm died before the end of the study. When exposed to 1.6 ppm, male rats did not gain weight and female rats showed decreased body weight; in the 0.5 and 0.16 ppm groups, body weight gain was normal. In the 1.6 and 5 ppm groups, breathing through the mouth, laboured breathing, ocular and nasal discharge, and rough fur were observed; no clinical signs were observed in rats exposed to 0.5 and 0.16 ppm. At necropsy, gross lesions were observed only in the animals that died or were moribund; these lesions included crusted exudate at the anterior tip of the nares, grey and thickened laryngeal mucous, and exudate or crust on the surface of the tongue. Exposure related histopathological lesions were found in the nasal passages and larynx of the rats exposed to concentrations from 0.5 ppm; the lesions included necrosis and acute (neutrophilic) inflammation of the respiratory and olfactory epithelium and hyperplasia and squamous metaplasia of the respiratory epithelium.
All mice exposed to concentrations from 1.6 ppm died or were moribund before the end of the study. Marked respiratory difficulties, breathing through the mouth, ocular and nasal discharge, and cessation of eating and drinking were observed in these animals. All mice exposed to 0.5 and 0.16 ppm survived the study and body weight gain was comparable to that observed with control mice. No clinical signs of toxicity were observed in these groups. At necropsy, red crust at the anterior nares and grey, thickened laryngeal mucous were observed in the mice of the 16.0 ppm group. Exposure related histopathologic lesions of the nasal passages were observed in mice exposed from 1.6 ppm and included minimal to mild necrosis and acute (neutrophilic) inflammation of the respiratory and olfactory epithelium and squamous metaplasia of the respiratory epithelium. Necrosis of the mucosa and squamous metaplasia of the respiratory epithelium of the larynx were also observed. Lesions of the trachea, consisting of inflammation and necrosis of the respiratory mucosa, were observed only in mice exposed to 16 ppm.
Groups of 10 male and 10 female Fischer 344 rats were exposed to 0, 0.2, 0.63, and 2.1 ppm glutaraldehyde (0, 0.8, 2.6, and 8.8 mg/m3) vapour for 6 hours/day for 9 days over an 11 day period. Sixteen rats exposed to 2.1 ppm and one rat exposed to 0.63 ppm died between days 3 and 9. Excess lacrimation, salivation, and nasal discharge were observed in rats from all treatment groups. Animals exposed to 0.63 and 2.1 ppm also had labored breathing. Loss of initial body weight and decreased feed consumption were observed in the mid- and high-dose groups. Decreased absolute weights for liver, lung, kidney, and testes were seen at 0.63 ppm. (Union Carbide Corporation 1983 - quoted from Ballantyne 1995).
Groups of 12 male and 12 female Fischer 344 rats were exposed to glutaraldehyde concentrations of 0.3, 1.1 and 3.1 ppm (1.3, 4.6, and 13 mg/m3). Over an 11-day period, the rats were exposed for 6 hours a day for 9 days. Mortality occurred in the 3.1 ppm group (13/24 animals). Signs of toxicity (audible breathing, labored breathing, mouth and abdominal breathing), body weight decreases, and food and water consumption decreases, were observed in a concentration-related manner at 1.1 and 3.1 ppm, whereas no effects on these parameters were observed at 0.3 ppm. Haematological and clinical changes as well as changes in organ weights at 1.1 and 3.1 ppm were probably related to body weights and hydration changes. Rhinitis, squamous metaplasia of the olfactory mucosa, and olfactory atrophy were clearly present at 1.1 and 3.1 ppm. At 0.3 ppm only rhinitis (10% of the animals) was seen. Light and electron microscopic examination of sciatic nerves from the 0.3 and 1.1 ppm groups showed no structural abnormalities. (Union Carbide Corporation 1983 - quoted from Ballantyne 1995).
For assessment of nasal toxicity, male Swiss OF1 mice (10 animals per group) were exposed to glutaraldehyde vapour for 6 hours/day, 5 days per week to concentrations of 0.3, 1.0, or 2.6 ppm (1.3, 4.2, 11 mg/m3) for 4, 9 or 14 days. Recovery was studied in 30 mice exposed to 1.0 ppm (4.2 mg/m3) for 14 days; groups of 10 mice were sacrificed 1, 2, or 4 weeks after the end of exposure. (Zissu et al. 1994).
In mice exposed to 1.0 and 2.6 ppm, respiratory difficulty (gasping, lung rales, and mouth breathing) was observed. The 2.6 ppm exposure was interrupted after 5 days because of the death of 4 of the 10 mice. In the 1.0 ppm group, mice showed marked excitation (nervously running around, abdominal swelling, rougher hair and unhealthier looking. In the 0.3 ppm group, no clinical signs of toxicity was observed. Lesions were observed in all exposed mice and affected exclusively the respiratory epithelium of the septum, the naso- and maxilloturbinates and also to a lesser extent the lateral wall, but not the olfactive one. The respiratory epithelium showed areas of deciliation, squamous metaplasia, focal cell damage with exfoliation and necrosis. The severity increased with glutaraldehyde concentration from 0.3 to 1.0 ppm and remained constant from 1.0 to 2.6 ppm, but it did not depend on exposure time. In mice exposed to 1.0 ppm for 14 days and sacrificed after a recovery period, the respiratory epithelium showed the same severe change after rest priods of 1 and 2 weeks, but changes were not so significant after a rest period of 4 weeks. None of the exposed mice showed any lesions in the lungs.
The sensory irritation of glutaraldehyde was determined in male Swiss OF1 mice (6 mice per group) by using the breathing frequency as an indeks. The breathing frequency was monitored before and during a 60-minute exposure period (oronasal exposure) to concentrations of 0.7, 1.3, 1.7, 3.2, 4.3, or 4.5 ppm (2.9, 5.5, 7.1, 13.4, 18, 19 mg/m3). The onset of the maximum response was rapid (within 2 minutes). The effect was steady during the exposure period for the low concentrations (0.7-1.7 ppm) but decreased in the response with time at the higher concentrations (3.2-4.5 ppm). After the exposure, recovery was rapid and concentration-dependent. The RD50 was calculated to 2.6 ppm (11 mg/m3). (Zissu et al. 1994).
In a respiratory irritation study, groups of 4 ND4 Swiss Webster mice were exposed to seven different glutaraldehyde vapour concentrations in the range of 1.6 to 36.7 ppm (6.7-154 mg/m3). Concentration related decreases in the respiratory rate was measured with a maximum at 3 to 20 minutes. The RD50-value was calculated to be 13.9 ppm (58 mg/m3). (Werley et al. 1995 - quoted from Beije & Lundberg 1997, Union Carbide Corporation 1994 - quoted from Ballantyne 1995).
The respiratory sensitising potential of glutaraldehyde vapour was studied in male Dunkin Hartley guinea pigs (8 animals per group) exposed for one hour per day for five consecutive days to an inducing concentration of 13.9 ppm (58 mg/m3). Challenge exposure to 4.4 ppm (18 mg/m3) at 14, 21, and 35 days after the final induction exposure did not produce any evidence of respiratory sensitisation. (Ballantyne 1995, Werley et al. 1995 - quoted from Beije & Lundberg).
Oral administration
LD50-values for glutaraldehyde in rats ranging from 2380 mg/kg for a 25% solution to 252 mg/kg for a 2% alkaline solution have been reported. Using the 2% solution, irritation of the gastro-intestinal tract with haemorrhagic irritation at larger doses was observed at necropsy. Congestion of the lungs and of the abdominal viscera was observed in many animals. (Smyth et al. 1962, Stonehill et al. 1963 - both quoted from CIR 1996).
In IUCLID (1996), LD50-values for rats ranged from 233-733 mg/kg for a 50% solution to 99-123 mg/kg for a 1% solution of glutaraldehyde. No experimental details were provided, so the studies can not be properly evaluated. A possible explanation for the higher toxicity of the less concentrated solutions could be that the more concentrated solutions may produce severe irritation and possibly corrosive effects to the gastrointestinal tract which may compound significantly with any systemic effects in determining acute peroral toxicity.
In mice, LD50-values ranged from 100-352 mg/kg (CIR 1996, IUCLID 1996).
For guinea pigs, an LD50-value of 50 mg/kg has been reported (IUCLID 1996).
Drinking water containing 0, 10, 100, or 1000 ppm glutaraldehyde was given to Fischer 344 rats (10 males and 10 females per group) for 14 days. Decreased feed and water consumption in males and decreased water consumption in the females were observed in the high-dose group. No other clinical signs of toxicity, alterations in serum chemistry and haematology, or effects on organ weights were observed. Histology revealed mild gastric mucosal gland hyperplasia. (Union Carbide Corporation 1985 - quoted from Ballantyne 1995).
Groups of CD-1 mice (10 males and 10 females per group) were given drinking water containing 0, 100, 250, or 1000 ppm glutaraldehyde for 14 days. Decreased water consumption was seen at 1000 ppm (both sexes) and at 250 ppm (males only). Decreased body weight gain occurred with males of the two highest dose groups. No other clinical signs of toxicity, alterations in serum chemistry and haematology, and gross or microscopic pathology were observed. Relative kidney weight was increased for 1000 ppm females. (Union Carbide Corporation 1988 - quoted from Ballantyne 1995).
Dermal contact
The LD50-values for rats and mice are given as >2000 and >4500 mg/kg, respectively. For rabbits, values ranging from 897 to >2825 mg/kg are given. (IUCLID 1996).
In a study to determine the potential for systemic toxicity by short-term repeated skin contact, groups of 5 male C3H/HeJ mice had 10 daily 50 ml applications of 0.05, 0.25, 0.5, 2.5, 5, 25, and 50% solutions of glutaraldehyde in water to the clipped dorsal trunk skin. Doses were applied for 5 consecutive days and, after a 2-day rest period, for a further 5 consecutive days. All mice receiving 25 or 50% solutions died within nine applications; no consistent necropsy findings were observed. Slightly decreased body weights were observed in mice receiving 5% solution. Skin irritation was seen at doses of 5% and higher. (Union Carbide Corporation 1981 - quoted from Ballantyne 1995).
Fisher 344 rats (10 males and 10 females per group) were given 20 epicutaneous applications of aqueous glutaraldehyde solutions over a 26-day period. Dosages used were 2.0 ml of 2.5, 5.0, or 7.5% solutions being equivalent to 50, 100, and 150 mg/kg b.w./day. Five consecutive daily 6-hour occluded applications were made to the clipped dorsal trunk skin each week for the 4-week treatment period. The control group received filtered water at the same dose volume. No signs of systemic toxicity were observed. Local skin irritation was minimal, mainly minor erythema and occasional edema. Pathological findings were limited to the treated areas of skin, and were dose-related. (Union Carbide Corporation 1994 - quoted from Ballantyne 1995).
Skin irritation
The skin irritating properties of glutaraldehyde have been studied in a number of tests with varying concentrations of glutaraldehyde. The best described study is given below.
A 0.5 ml dose of 1, 2, 5, 10, 25, 45, and 50% aqueous glutaraldehyde solutions was applied under an occlusive dressing for 4 hours to the shaven dorsal trunk skin of groups of rabbits (6 animals per group), and the treatment sites were observed for 21 days. A dose-response relationship was observed for severity and duration of inflammatory effects. The 45 and 50% solutions produced moderately severe inflammation, with full-thickness necrosis of the skin. With the 25% solution, moderate inflammation was present, but less marked than with the higher concentrations. Minor to moderate inflammation occurred with solutions of 1-10%. The threshold for skin irritation was around 1%. (Ballantyne 1995).
Eye irritation
An alkaline 2% glutaraldehyde solution (0.1 ml) was instilled into the conjunctival sac of one eye of each of 12 rabbits. The eyes of 3 of the rabbits were rinsed 30 seconds later. Severe corneal opacity and irritation of the iris and conjunctiva were observed in unrinsed eyes during the 7-day observation period. Irritation of the conjunctiva, which was similar in rinsed eyes, also lasted 7 days. However, irritation of the iris and cornea was less in rinsed eyes than in unrinsed eyes, and recovery was partial. The 2% alkaline glutaraldehyde solution was thus a severe eye irritant. (Miner et al. 1977 - quoted from CIR 1996 and from Beije & Lundberg 1997).
Differing volumes, in the range of 0.005 to 0.1 ml of aqueous glutaraldehyde solutions from 0.1 to 45% were instilled into the eyes of rabbits. Eyes were inspected periodically thereafter, up to 3 weeks, for signs of ocular and periocular injury and inflammation. There was a clear dose-response relationship for corneal injury and conjunctivitis. The lowest concentration producing transient minor corneal injury (at 0.1 ml) was 1.0%, and the no-effect concentration was 0.1%. At higher concentrations, corneal injury became more marked and prolonged, being severe at 5% (0.1 ml). The threshold for conjunctival irritation was 0.2%, and the no-effect concentration was 0.1%. (Ballantyne 1995).
Sensitisation
According to IUCLID (1996), various skin sensitisation tests have been carried out. Of these the closed patch test, the skin painting test, the open epicutaneous tests, and the mouse ear swelling test were positive, while the Buehler test was negative. No Guinea Pig Maximisation Test was included. In the two open epicutaneous tests, one in mice and one in guinea pigs, a dose related contact hypersensitivity was observed.
A contact hypersensitivity study has been conducted in female albino Hartley strain guinea pigs and female B6C3F1 mice. Induction concentrations were 0.3, 1.0, and 3.0% glutaraldehyde, and challenge was with 10% glutaraldehyde. Guinea pigs received 100 µl induction doses by direct skin application for 14 consecutive days, and mice received 20 µl epicutaneously for 5 or 14 consecutive days. Rest periods before challenges were 7 or 14 days for guinea pigs, and 4 or 7 days for mice. Measurement of contact hypersensitivity was by both visual evaluation and a radioisotopic assay. Both guinea pigs and mice showed an applied dosage dependent contact hypersensitivity response. A significant response was seen in mice at 0.3% and in both species at 1 and 3%. (Stein 1989 - quoted from Beije & Lundberg and from Ballantyne 1995).
4.2 Long term toxicity
Inhalation
In a subchronic inhalation study, F344/N rats and B6C3F1 mice (10 animals of each sex per group) were exposed to glutaraldehyde by whole-body inhalation at concentrations of 0, 62.5, 125, 250, 500, and 1000 ppb (0, 0.26, 0.53, 1.1, 2.1, 4.2 mg/m3) for 6.5 hours per day, 5 days a week for 13-weeks. In addition to histopathology, evaluations included clinical pathology and assessments of sperm morphology and oestrous cycle length. (NTP 1993 - quoted from Gross et al. 1994, Beije & Lundberg 1997, CIR 1996, and from Ballantyne 1995).
No exposure-related deaths occurred in rats, whereas all mice exposed to 1000 ppb and two females exposed to 500 ppb died before the end of the study. Mean final body weights and body weight gains were significantly lower for rats exposed to 1000 ppb; lower body weight gain was also noted for females exposed to 500 ppb. In mice, mean final body weight was reduced in a concentration-related manner, and was significant in the 250- and 500-ppb groups. There was no clear evidence of systemic toxicity in rats or mice by histopathologic or clinical pathology assessments. Treatment-induced lesions (including epithelial erosions, inflammation, and squamous metaplasia) were confined to the anterior third of the nose and were present in both sexes and species. In rats, lesions were most extensive in rats exposed to 1000 ppb, but were also noted in the 250- and 500-ppb groups, and in one male exposed to 125 ppb. In mice, lesions were most severe in animals in the 1000-ppb group, but were noted at concentrations down to 62.5 ppb. Mice appeared to be more sensitive than rats. The NOAEL was 125 ppb for respiratory lesions in rats. A NOAEL was not reached for mice as effects were noted at the lowest exposure level of 62.5 ppb.
According to Gross et al. (1994), the lesions induced by glutaraldehyde were more anterior in the nose than those produced by formaldehyde, they differed in character, and no evidence of "pre-neoplastic" lesions or karyomegaly, as reported for formaldehyde, was observed with glutaraldehyde.
In another study, Fischer 344 rats (20 males and 20 females per group) were exposed to glutaraldehyde vapour concentrations of 20.8, 49.3, or 194.2 ppb (0.09, 0.2, 0.8 mg/m3) for 6 hours per day, 5 days a week for 14 weeks. Decreased body weight and minor signs of ocular and nasal irritation were observed at 49.3 and 194.2 ppb; at 20.8 ppb there was only minor transient body weight decrease. There were no biochemical or morphological indications of any specific organ toxicity. (Union Carbide Corporation 1982 - quoted from Ballantyne 1995).
Oral administration
rats
Groups of three rats were given drinking water containing 0, 0.05, 0.1, or 0.25% of glutaraldehyde for 11 weeks. All of the animals had a "largely normal" rate of weight gain and appeared normal clinically. The rats were killed at the end of the experiment and nervous system tissue was examined microscopically. No signs of adverse effects were found. (Spencer et al. 1978 - quoted from CIR 1996 and from Beije & Lundberg 1997).
Fischer 344 rats (numbers per group not stated) were given 0, 50, 250, or 1000 ppm glutaraldehyde in the drinking water for 13 weeks. An additional 10 animals of each sex were added to the control and high concentration groups as recovery animals and sacrificed 4 weeks after the 13-week dosing period. The daily intakes were given as 5, 25, and 100 mg/kg b.w. per day for males and 7, 35, and 120 mg/kg b.w. per day for females. A dosage-related decreased food and water consumption was observed at 250 and 1000 ppm. Body weights and body weight gains were reduced in high-dose males during the treatment period, with partial recovery in the 4-week postdosing period. A dose-related increase in absolute and relative kidney weights in females, and relative kidney weights in males was present at 13 weeks in the 250 and 1000 ppm groups; values were similar to those of the controls at the end of the recovery period. There was no evidence, morphological or biochemical, for systemic tissue or organ toxicity. The dose of 50 ppm was a no-effect level in this study. (Union Carbide Corporation 1985 - quoted from Ballantyne 1995).
In a 2-year carcinogenicity study, Fischer 344 rats (100 males and 100 females per group) received 0, 50, 250 or 1000 ppm glutaraldehyde in the drinking water. Interim sacrifices (10 males and 10 females of each group) were performed at 52 and 78 weeks after the start of dosing. Based on the water consumption values, the daily intake of glutaraldehyde was calculated to 3.6, 17.1, and 63.9 mg/kg b.w. per day for males and 5.5, 25.1, and 85.9 mg/kg b.w. per day for females of the 50, 250, and 1000 ppm groups, respectively. (Union Carbide Corporation 1994 - quoted from Ballantyne 1995).
None of the dosages had any effect on mortality or survival. There were dose-related decreases in drinking water consumption for mid- and high-dose males and females. A reduced food consumption was observed at 1000 ppm and a trend to reduced food consumption at 250 ppm. Dose-related decreases in absolute body weight and body weight gain were seen in males (250 and 1000 ppm) and in females (1000 ppm). Absolute and relative kidney weights were increased in all males and in mid- and high-dose females. Gastric irritation was significantly greater in high-dose animals compared with the controls. Tubular pigmentation and basophilia were seen in the kidneys of males (1000 ppm) and females (250 and 1000 ppm); according to the author, these lesions were probably related to hemolytic changes associated with large granular lymphocytic leukaemia (LLGL), see 4.5.
mice
CD-1 mice (numbers per group not stated) were given 0, 100, 250, or 1000 ppm glutaraldehyde in the drinking water for 13 weeks. An additional 10 animals of each sex were added to the control and high concentration groups as recovery animals and sacrificed 6 weeks after the 13-week dosing period. The daily intake was given as 25, 61 and 200 mg/kg b.w. per day for males, and 31, 74, and 238 mg/kg b.w. per day for females. Water consumption was reduced in the high-dose group, but returned to control values over the recovery period. Relative kidney weight was increased in high-dose females at the end of the dosing period but not at the end of the recovery period. There was no evidence for any target organ or tissue systemic toxicity. (Union Carbide Corporation 1985 - quoted from Ballantyne 1995).
dogs
Beagle dogs (2 males and 2 females per group) were given glutaraldehyde in the drinking water at concentrations of 0, 50, 150, or 250 ppm for 13 weeks. The daily intake was given as 3.3, 9.6, and 14.1 mg/kg b.w. per day for males, and 3.2, 9.9, and 15.1 mg/kg b.w. per day for females. Vomiting was seen in the 150 and 250 ppm groups. A trend for reduced water consumption were seen in males (150 and 250 ppm) and in females (250 ppm) for the first 6 weeks of dosing. There was no evidence for any target organ or tissue systemic toxicity. (Union Carbide Corporation 1990 - quoted from Ballantyne 1995).
Dermal contact
A 0.5 ml dose of a 2% alkaline glutaraldehyde solution was applied daily for 6 weeks to the closely clipped skin of albino rabbits. The solution was spread with a brush and allowed to dry. No evidence of systemic toxicity was observed. (Stonehill et al. 1963 - quoted from CIR 1996).
4.3 Reproductive / developmental effects
In the 13-week study of NTP (reported in section 4.2), no adverse effects were observed on sperm morphology and vaginal cytology in F344/N rats exposed to up to 1000 ppb (4.2 mg/m3) glutaraldehyde vapour. In B6C3F1 female mice, the 250 and 500 ppb groups (1.1 and 2.1 mg/m3) spent more time in oestrus and dioestrus and less time in metoestrus and prooestrus (500 ppb only) than the control animals. There were no effects in any of the reproductive parameters studied in male mice. (NTP 1993 - quoted from CIR 1996).
In a two-generation study, CD rats (28 males and 28 females per group) were given 0, 50, 250, or 1000 ppm glutaraldehyde in the drinking water for 10 weeks prior to mating and throughout mating, gestation, and lactation. After weaning, the F1 generation was founded using 28 males and 28 females. These animals were given the same concentration of glutaraldehyde as their parents for 10 weeks prior to mating and throughout mating, gestation, and lactation. Parental animals and ten weanlings of each sex and dose group were necropsied. (Bushy Run Research Center 1994 - quoted from CIR 1996, Union Carbide Corporation 1994 - quoted from Ballantyne 1995).
In the 250 and 1000 ppm groups, there was a consistent reduction in water consumption. During gestation and lactation, water consumption remained reduced in the dams of the 250 and 1000 ppm dose groups. Reduced food consumption was observed in the high-dose females near the end of gestation. No effects on mating, fertility, gestational factors, litter size and sex ratio were observed. In the 1000 ppm F1 and F2 pups, body weights and body weight gains were reduced.
Pregnant Wistar rats (25 animals per group) received 0, 50, 250, or 750 ppm glutaraldehyde in the drinking water from day 6 to 16 of gestation in a study carried out according to OECD guideline 414 and GLP. The daily intake of glutaraldehyde was estimated to 5, 26, and 68 mg/kg b.w. per day for the 50, 250, and 750 ppm dose groups, respectively. In the 750 ppm group, intake of drinking water was clearly reduced from day 6 to 16 and in the 250 ppm group, the drinking water intake was slightly reduced from day 10 to 15. No embryo- or foetotoxic and no teratogenic effects were seen at any doses. (IUCLID 1996, Union Carbide Corporation 1990 - quoted from Ballantyne 1995).
Pregnant Himalayan rabbits (15 animals per group) received glutaraldehyde once daily by gavage (in water) from day 7 to 19 of gestation at dosages of 5, 15, and 45 mg/kg b.w. per day in an OECD guideline 414 and GLP study. The control group was given distilled water. (IUCLID 1996, Union Carbide Corporation 1990 - quoted from Ballantyne 1995).
In the high dose group, 5 out of 15 animals died. The food intake was clearly reduced during the dosing period and the day after. Maternal body weight was clearly reduced from day 11 to 29. Diarrhoea and/or congestion was observed. At necropsy, irritation of the gastrointestinal tract, reduced uterus weight, and increased number of postimplantation losses were observed. Nine out of ten surviving does had no live foetuses. Trend for reduced placental weights and reduced foetal body weight were observed. In the the lower dose groups, no signs of maternal or developmental toxicity were observed.
4.4 Genotoxic effects
Glutaraldehyde was mutagenic in Salmonella typhimurium strains TA100, TA102, and TA104 with and without S9 metabolic activation as well as in mouse L5178Y lymphoma cells in the absence of S9. It induced sister chromatid exchanges in Chinese hamster ovary cells with and without S9. In one laboratory, chromosomal aberrations were induced in Chinese hamster ovary cells in the absence of S9 only; no increase in chromosomal aberrations was observed with or without S9 in a second laboratory. Glutaraldehyde did not induce sexlinked recessive lethal mutations in germ cells of male Drosophila melanogaster treated as adults by feeding or injection or treated as larvae by feeding. (NTP 1993 quoted from IUCLID 1996).
In CIR (1996), review results of more than 30 in vitro genotoxicity tests using glutaraldehyde are given. Most of these tests are from the early 1980ties. In all, 12 of these tests were reported as positive.
In view of the fact that most of the in vitro tests carried out by NTP were positive, glutaraldehyde should be considered a positive genotoxicant in vitro.
Only two reports on the in vivo genotoxicity of glutaraldehyde are given in the CIR (1996) review. These were negative (unscheduled DNA synthesis (UDS) in male rat hepatocytes isolated following oral administration). (Mirsalis et al. 1989, 1985 - quoted from CIR 1996).
In IUCLID (1996), a mouse dominant lethal assay (using doses of 0, 30, or 60 mg/kg b.w.), a micronucleus test in mice (using gavage dosing of 80, 160, or 250 mg/kg b.w. and exposure for 24, 48, or 72 hours), a rat UDS test (using gavage dosing of 30, 150, or 600 mg/kg b.w. and exposure for 2 or 12 hours), and a rat bone marrow chromosome aberration test (using gavage dosing of 25, 60, or 120 mg/kg b.w. to males and 15, 40, or 80 mg/kg b.w. to females and exposure periods of 12, 24, and 48 hours) are reported as negative in vivo genotoxicity tests.
4.5 Carcinogenic effects
In a 2-year carcinogenicity study, Fischer 344 rats (100 males and 100 females per group) received 0, 50, 250 or 1000 ppm glutaraldehyde in the drinking water. Based on the water consumption values, the daily intake of glutaraldehyde was calculated to 3.6, 17.1, and 63.9 mg/kg b.w. per day for males and 5.5, 25.1, and 85.9 mg/kg b.w. per day for females of the 50, 250, and 1000 ppm groups, respectively. (Union Carbide Corporation 1994 - quoted from Ballantyne 1995).
For general toxicity, see 4.2. The major finding was an increase in large granular lymphocytic leukaemia (LGLL) in all female dose groups. The incidence of LGLL in spleen and liver is given below:
| Sex | 0 ppm | 50 ppm | 250 ppm | 1000 ppm | |
| Spleen | M F |
43 24 |
51 41* |
40 41* |
46 53* |
| Liver | M F |
37 23 |
48 40* |
39 40* |
45 52* |
* statistically significant.
The increase in LGLL was statistically significant for the females of all dosed groups, but not for the males. LGLL is a commonly occurring spontaneous neoplasm in Fischer 344 rats. According to the author "the incidence of LGLL in control Fischer 344 rats varies between different laboratories, and within the same laboratory. Thus, whilst the average incidence of LGLL in female Fischer 344 rats is usually cited as 24-27%, the individual incidence in some conducted studies has been reported up to 52% in control female groups. In a previous study from the same laboratory, the incidence of LGLL in control groups of male and female Fischer 344 rats were, respectively, 66 and 44%." With respect to the interpretation of the increased incidence of LGLL in females, the author stated "that it appears unlikely that the increase in LGLL in female rats is due to a direct chemical carcinogenic effect, but was a result of the chronic administration of glutaraldehyde in drinking water having a modifying influence on one or more of the factors influencing the expression of this spontaneously occurring neoplasm in the female Fischer 344 rats".
A carcinogenicity study (inhalation) on rats and mice performed by the NTP has not yet been finalised (Beije & Lundberg 1997).
5. Regulations, limit values
| Ambient air | - |
| Drinking water | - |
| Soil | - |
| OELs | Denmark: 0.2 ppm (0.8 mg/m3) (At 1996) |
| Classification | Glutaraldehyde is classified for acute toxic effects (T;R23/25 - toxic by inhalation and if swallowed), for corrosive effects (C;R34 - causes burns), for sensitisation (R42/43 - may cause sensitisation by inhalation and skin contact), and for effects on the environment (N;R50 - very toxic to aquatic organisms) (MM 1997). |
| IARC/WHO | - |
| US-EPA | - |
6. Summary
Description
Glutaraldehyde (pure form) is a colourless, oily liquid. Commercial solutions often have an amber tint and an odour similar to spoiled fruit. Glutaraldehyde is miscible with water. It has a low vapour pressure.
Environment
Glutaraldehyde does not appear to occur naturally. No data on ambient levels as well as fate processes have been found.
Human exposure
The most probable routes of human exposure to glutaraldehyde are dermal contact and inhalation by workers involved in the manufacture and use of the substance.
Toxicokinetics
No specific data on absorption and distribution have been found. Material balance studies in rats and mice indicate that the majority of a dermal dose remains at the application site. The metabolism of glutaraldehyde has not been studied in detail but it has been suggested that glutaraldehyde is oxidised through acidic intermediates to carbonmonoxide which is expired.
Human toxicity
Several studies on hospital personnel indicate that vapours of glutaraldehyde causes irritation of eyes, respiratory tract, throat, and skin as well as nausea and headache at exposure levels ranging from 0.02 to 0.8 mg/m3. One study has reported irritative symptoms at 0.8 mg/m3 but not at 0.4 mg/m3. Case studies indicate that glutaraldehyde also may cause occupational asthma; in one study, seven of eight workers had positive specific bronchial challenge tests to glutaraldehyde (mean short-term air concentration was 0.16 mg/m3, mean concentration during the challenge test was 0.068 mg/m3). Glutaraldehyde solutions may cause mild to severe irritation of the skin, depending on the concentration of the solution and the duration of exposure. Several case reports and studies on allergic contact dermatitis indicate that glutaraldehyde is a skin sensitiser.
Animal toxicity
LC50-values (4 hours exposure) ranging from 0.1 to 0.8 mg/l have been reported for rats. Short-term studies (up to 2 weeks) have shown histopathological lesions (necrosis, inflammation, hyperplasia, squamous metaplasia) of the nasal passages and larynx of rats (from 2 mg/m3) and mice (from 1 mg/m3). One study on mice showed no recovery of the histopathological lesions two weeks after exposure (4 mg/m3), whereas partial recovery was observed four weeks after exposure; none of the exposed mice (1-11 mg/m3) showed any lesions in the lungs. RD50-values of 11 and 58 mg/m3 have been reported in mice. One study indicate that glutaraldehyde is not a respiratory sensitisator in rats. In 13-week inhalation studies of rats and mice, the NOAEL for respiratory lesions in rats was 0.5 mg/m3 and the LOAEL in mice was 0.26 mg/m3 (the lowest dose in the study). The lesions observed were of a similar kind as those caused by formaldehyde, although they were more anterior in the nose than those reported for formaldehyde. No evidence of systemic toxicity (histopathological or clinical pathology assessments) was observed.
Following oral administration of glutaraldehyde in drinking water to rats, decreased food and water consumption, reduced body weight and body weight gains, and increased kidney weight (absolute and relative) were observed; a NOAEL of 50 ppm (around 5 mg/kg b.w./day) was observed. Mice and dogs appeared to be less sensitive than rats. No evidence for systemic tissue or organ toxicity was observed in these studies.
The threshold for skin irritation was around 1% and for eye irritation around 0.1% when glutaraldehyde was tested in aqueous solution. In 4 out of 5 different skin sensitisation tests, not including the guinea pig maximisation test, glutaraldehyde was positive.
Reproductive and developmental effects
No human data on reproductive effects have been found; one study did not indicate an increased risk of spontaneous abortions or foetal malformations. No effects on fertility or developmental parameters was observed in a two-generation study of rats or in teratology studies of rats and rabbits.
Genotoxicity
Glutaraldehyde has shown a genotoxic potential in vitro causing mutations in both bacterial and mammalian cells, and sister chromatid exchanges and chromosomal aberrations in mammalian cells. However, five in vivo tests (two tests in rats for unscheduled DNA synthesis, a mouse dominant lethal assay, a micronucleus test in mice, and a rat bone marrow chromosome aberration test) have shown negative results.
Adducts have been formed on reaction of glutaraldehyde with components of DNA (deoxyadenosine, deoxyguanosine and deoxycytidine, but not deoxythymidine).
Carcinogenicity
In a mortality study of workers (exposed to concentrations up to 0.7 mg/m3), no evidence of increased mortality and cancer was observed.
A carcinogenesis study (inhalation) on rats and mice has not yet (1997) been finalised. In an oral carcinogenicity study on rats (drinking water), an increased incidence of large granular lymphocytic leukaemia in spleen and liver was observed.
7. Evaluation
Several studies on hospital personnel indicate that glutaraldehyde as vapour causes irritation of eyes, respiratory tract, and throat as well as nausea and headache at exposure levels ranging from 0.1 to 0.8 mg/m3. Case studies indicate that glutaraldehyde also may cause occupational asthma; in one study, mean short-term air concentration was 0.16 mg/m3 and mean concentration during the challenge test was 0.068 mg/m3. Direct skin contact with glutaraldehyde solutions can irritate and affect the skin. Glutaraldehyde is a recognised skin sensitiser. In a mortality study of workers (exposed to concentrations up to 0.7 mg/m3), no evidence of increased mortality and cancer was observed.
Data from several short-term and long-term animal studies (rats, mice and dogs) indicate that glutaraldehyde is very reactive when inhaled or when applied dermally as clear evidence of irritation and histopathological changes have been observed at the site of initial contact. No lesions have been observed in the lungs of mice and no evidence of systemic toxicity (histopathological or clinical pathology assessments) has been seen in rats, mice, or dogs. In 4 out of 5 different skin sensitisation tests, not including the guinea pig maximisation test, glutaraldehyde was positive.
The studies available on reproductive and developmental effects indicate that glutaraldehyde is neither a reproductive toxicant nor a teratogenic substance. The tests on genotoxicity show equivocal results with in vitro tests being positive and in vivo tests being negative. The lack of in vivo genotoxic effects suggests that glutaraldehyde does not pose a genotoxic risk to humans and animals. A carcinogenesis study (inhalation) on rats and mice has not yet (1997) been finalised. In an oral carcinogenicity study on rats (drinking water), an increased incidence of large granular lymphocytic leukaemia in spleen and liver was observed, however, this type of leukaemia does not have a predictive value for the occurrence of cancer in humans.
Based on the available human data as well as the studies on experimental animals, the critical effects following inhalation exposure to vapours of glutaraldehyde are considered to be the irritative effects on eyes, respiratory tract, and throat, development of occupational asthma as well as the histopathological lesions observed in the nasal passages of rats and mice. These lesions were of a similar kind as those caused by formaldehyde, although they were more anterior in the nose than those reported for formaldehyde.
The question whether long-term exposure to glutaraldehyde may lead to development of nasal cancer, as reported for formaldehyde, may await the results of the NTP study. However, the animal studies on formaldehyde show that there seems to be a threshold for the development of tumours in the nasal cavities as tumours were only found at exposure levels at which clear cytotoxic effects occur. These data on formaldehyde thus indicate that cytotoxicity is a prerequisite for the development of nasal cancer.
Based on the similarities in the nasal toxicity of formaldehyde and glutaraldehyde it is considered that protection towards the irritative effects also will protect against development of nasal lesions and cancer.
Irritation of eyes, respiratory tract, and throat has been observed at exposure levels ranging from 0.1 to 0.8 mg/m3; one study on occupational asthma showed positive effects at mean short-term air concentration of 0.16 mg/m3 and mean concentration during the challenge test of 0.068 mg/m3. Thus, the human data on irritative effects as well as on development of occupational asthma do not point to a clear NOAEL or LOAEL for exposure to glutaraldehyde. For the purpose of estimating a limit value in air, an overall LOAEL of 0.1 mg/m3 for irritative effects and development of occupational asthma is considered. This LOAEL is supported by the data from animal studies where a NOAEL for respiratory lesions in rats was 0.5 mg/m3 and a LOAEL in mice was 0.26 mg/m3. The odour threshold in air has been reported to be 0.16 mg/m3.
8. Limit value in air
For the estimation of a limit value, an overall LOAEL of 0.1 mg/m3 for irritative effects on eyes, respiratory tract, and throat and for development of occupational asthma is considered.

= 0.001 mg/m3
The safety factor SFI is set to 1 as human are used. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 10 because of using a LOAEL in stead of a NOAEL, because of the uncertainty in establishing a LOAEL, and because a NOAEL for development of occupational asthma are not available.
9. C-value
A limit value of 0.001 mg/m3 has been calculated. For substances having acute or subchronic effects, but for which activity over a certain period of time is necessary before the harmful effect occurs, the C-value is set at the limit value. A C-value of 0.001 mg/m3 and placing in Main Group 1 is proposed.
Glutaraldehyde is to be placed in Main Group I, because of the similarities in the toxicity between glutaraldehyde and formaldehyde, which is placed in Main Group 1.
C-value
0.001 mg/m3, Main Group 1.
10. References
ACGIH (1991). Glutaraldehyde. In: TLV's Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 1991-1992. Cincinnati, OH, 703-704.
At (1996). Grænseværdier for stoffer og materialer. Arbejdstilsynets At-anvisning Nr. 3.1.0.2, december 1996.
Ballantyne B (1995). Toxicology of glutaraldehyde. Review of studies and human health effects. Union Carbide Corporation.
Beauchamp RO, St. Clair MBG, Fennell TR, Clarke DO and Morgan KT (1992). A critical review of the toxicology of glutaraldehyde. Crit Review Toxicol 22, 143-174.
Beije B and Lundberg P (1997). Glutaraldehyde. DECOS and NEG Basis for an occupational standard. Arbete och Hälsa 20, 1-30.
CIR (1996). Final report on the safety assessment of glutaral. Cosmetic Ingredient Review. J Am Coll Toxicol 15, 98-139.
Corrado OJ, Osman J and Davies RJ (1986). Asthma and rhinitis after exposure to glutaraldehyde in endoscopy units. Human Toxicol 5, 325- 32X.
Frantz SW, Beskitt JL, Tallant MJ, Futrell JW and Ballantyne B (1993). Glutaraldehyde: species comparisons of in vitro skin penetration. J Toxicol - Cut & Ocular Toxicol 12, 349-361.
Gannon PFG, Bright P, Campbell M, O’Hickey SP and Burge PS (1995). Occupational asthma due to glutaraldehyde and formaldehyde in endoscopy and X-ray departments. Thorax 50, 156-159.
Gross EA, Mellick PW, Karl FW, Miller FJ and Morgan KT (1994). Histopathology and cell replication responses in the respiratory tract of rats and mice exposed by inhalation to glutaraldehyde for up to 13 weeks. Fundam Appl Toxicol 23, 348-362.
IUCLID (1996). Data sheet dated 07 Feb 1996.
McKelvey JA, Garman RH, Anuszkiewicz CM, Tallant MJ and Ballantyne B (1992). Percutaneous pharmacokinetics and material balance studies with glutaraldehyde. J Toxicol - Cut & Ocular Toxicol 11, 341-367.
Merck Index (1996). Glutaraldehyde. 12th. Ed., Rahway, New Jersey, Merck & Co., Inc., 4483.
MM (1997). The Statutory Order from the Ministry of the Environment no. 829 of November 6, 1997, on the List of Dangerous Chemical Substances.
Norbäck D (1988). Skin and respiratory symptoms from exposure to alkaline glutaraldehyde in medical services. Scand J Work Environ Health 14, 366-371.
Teta MJ, Avashia BP, Cawley TJ and Yamin AT (1995). Absences of sensitizations and cancer increases among glutaraldehyde workers. Toxic Substance Mechanisms 14, 293-305.
Zissu D, Gagnaire F and Bonnet P (1994). Nasal and pulmonary toxicity of glutaraldehyde in mice. Toxicol Lett 71, 53-62.
January 1996 / final
Evaluation of health hazards by exposure to
Furfural
and estimation of limit values in ambient air, soil and drinking water.
Elsa Nielsen
The Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
Denmark
1. General description
1.1 Identity
Molecular formula: C5H4O2
Structural formula:
| Molecular weight: | 96.08 |
| CAS-no.: | 98-01-1 |
| Synonyms: | 2-Furaldehyde 2-Furancarboxaldehyde 2-Furfuraldehyde 2-Formylfuran Furfurol |
1.2 Physical / chemical properties
| Description: | Pure furfural is a clear, colourless, oily liquid with a pungent, aromatic odour resembling that of bitter almonds. Turns yellow to brown on exposure to air and light. |
| Purity: | Furfural is available commercially at a purity >98%. |
| Melting point: | -36.5° C |
| Boiling point: | 161.8° C (at 760 mmHg) |
| Density: | 1.1598g/ml (at 20° C) |
| Vapour pressure: | 1 mmHg (133 Pa) at 20° C |
| Concentration of saturated vapours: | 0.13% at 20° C and 760 mmHg |
| Vapour density: | 3.31 (air = 1) |
| Conversion factor: | 1 ppm = 4.0 mg/m3 20° C 1 mg/m3 = 0.25 ppm 1 atm |
| Flash point: | 60° C (closed cup), 68 ° C (open cup) |
| Flammable limits: | 2.1 - 19.3 (v/v% in air) |
| Autoignition temp.: | 392° C |
| Water Solubility: | 83 g/l (at 20° C). Miscible with most of the common organic solvents, only slightly miscible with saturated aliphatic hydrocarbons. |
| logPoctanol/water: | 0.41 |
| Henry’s constant: | - |
| pKa-value: | - |
| Stability: | Furfural is very thermally stable in the absence of oxygen. |
| Incompatibilities: | Can react with oxidising materials. A resinification of almost explosive violence can occur upon contact with strong mineral acids or alkalies. |
| Odour threshold, air: | 0.078 ppm (Amoore & Hautala 1983). |
| Odour threshold, water: | 3.5 mg/l (Amoore & Hautala 1983). |
| References: | Merck Index (1976), ACGIH (1991), HSDB (1995), Brabec (1994), Kirk-Othmer (1985), A&H (1984), IARC (1995), NTP (1990). |
1.3 Production and use
Furfural is prepared industrially from pentosans present in cereal straws and brans by hydrolysis and dehydration with strong inorganic acids (IARC 1995, Brabec 1994, NTP 1990).
Furfural is extensively used in solvent extraction in the petroleum refining industry. It is also used as a solvent (for nitrated cotton, cellulose acetate and gums), to accelerate vulcanisation, as an ingredient of phenolic resins, as an intermediate in the synthesis of furan derivatives, as a weed killer, and as a fungicide. Furfural is also used as a flavouring agent. (IARC 1995, Brabec 1994, ACGIH 1991, NTP 1990, A&H 1984).
1.4 Environmental occurrence
Furfural is a volatile component of a wide range of fruits and vegetables (IARC 1995).
Air
Furfural has been identified as one of the main components of smoke condensates of pine and cottonwood. It was a major constituent of glowing fires of conifer logs. Residential burning of brown-coal briquettes led to emission of furfural, at an emission factor of 1.63 mg/kg. The estimated total amount of furfural emitted in the city of Leipzig was 530 kg/year. Furfural has been measured at a concentration of 0.19 mg/m3 at the foot of Mount Everest in Nepal. (IARC 1995).
Water
No data have been found.
Soil
No data have been found.
Foodstuffs
Furfural is formed during the thermal decomposition of carbohydrates and is thus found in numerous processed food and beverage products. It is also carried over into food from its use as an extraction solvent or as a component of flavour mixtures. The highest reported concentrations were found in wheat bread (0.8-14 mg/kg), cognac (0.6-33 mg/kg), rum (22 mg/kg), malt whisky (10-37 mg/kg), port wine (2-34 mg/kg), and coffee (55-255 mg/kg). The concentrations of furfural in juices were 0.01-4.94 mg/kg. (IARC 1995, JECFA 1993).
1.5 Environmental fate
No data have been found.
1.6 Human exposure
Food additive or processing use provides an intake estimated to be no more than 0.5-1% of the intake from other food sources (JECFA 1993).
No other data concerning human exposure have been found.
2. Toxicokinetics
2.1 Absorption, distribution
Furfural is extensively absorbed in humans after inhalation and in rats after oral administration (IARC 1995).
In a series of experiments, humans (healthy male volunteers aged 30-55 years) were exposed to 7 to 30 mg/m3 furfural vapour for a period of 8 hours. Inspired and expired air was analysed and pulmonary retention was calculated to be 78% (75-82%) and was not related to the level or duration of exposure. (Flek & Sedivec 1978).
Furfural vapour was also absorbed percutaneously. The amount absorbed by the skin corresponded to about 20-30% of the dose retained in the lungs. However, it was rather variable and increased with rising temperature and relative humidity. (Flek & Sedivec 1978).
After administration by gavage of [carbonyl-14C]furfural to rats at single doses of 0.127, 1.15 or 12.5 mg/kg in corn oil, 86-89% the dose was absorbed. After 72 h, high concentrations of radiolabel were found in liver and kidney, brain had the lowest concentration. The concentrations in liver and kidney were approximately proportional to the dose. (Nomeir et al 1992 - quoted from IARC 1995).
2.2 Elimination
The major route of metabolism of furfural is oxidation to furoic acid, followed by conjugation with glycine to furoylglycine. A minor pathway involves condensation of furoic acid with acetate to form furanacrylic acid, followed by conjugation with glycine to furanacryluric acid. The metabolites are excreted primarily via the kidneys into the urine.
In humans, the main metabolite in the urine was furoylglycine with slight amounts of furanacryluric acid. No free furoic acid was found in urine from exposed persons. The biological half-life was estimated to be 2-2.5 hours. (Flek & Sedivec 1978).
After administration by gavage of [carbonyl-14C]furfural to rats at single doses of 0.127, 1.15 or 12.5 mg/kg in corn oil, more than 60% of the dose was excreted after 12 h, reaching a plateau after 24 h. The major route of excretion was urine, which contained 83-88% of the dose. About 7% of a dose of 12.5 mg/kg was exhaled as 14C-carbon dioxide, and 2-4% of the dose was detected in the faeces. Furoylglycine was the major urinary metabolite (73-80% of dose), and furanacrylic acid (3-8%) and furoic acid (1-6%) were minor metabolites. The extent and rate of excretion of furfural metabolites were unaffected by dose. (Nomeir et al 1992 - quoted from IARC 1995).
2.3 Toxicological mechanisms
No data have been found.
3. Human toxicity
3.1 Short term toxicity
The main effect of furfural in humans has been reported to be skin and mucous membrane irritation.
In a NIOSH study of a grinding wheel plant, it was noted that furfural vapour in concentrations of 5 to 16 ppm (20-64 mg/m3) caused irritation of the eyes and respiratory passages (NIOSH 1975 - quoted from ACGIH 1991, A&H 1984).
In an old report, factory workers complained of headache and irritations to the throat and eyes. Air concentrations were reported to be about 2-14 ppm (8-56 mg/m3). (Korenman & Resnik 1930 - quoted from Brabec 1994, ACGIH 1991, A&H 1984).
3.2 Long term toxicity
No data have been found.
3.3 Reproductive and developmental effects
No data have been found.
3.4 Mutagenic and genotoxic effects
Six workers exposed to furfural and furfuryl alcohol in a furoic resin plant showed no significant difference in sister chromatid exchange frequency in peripheral blood lymphocytes in comparison with six control individuals (Gomez-Arroyo & Souza 1985 - quoted from IARC 1995 and JECFA 1993).
3.5 Carcinogenic effects
No data have been found.
4. Toxicity, animal data
4.1 Short term toxicity
Inhalation
single exposure
For rat and dog, LC50-values of 175 ppm and 370 ppm (700 and 1480 mg/m3) for 6 hours exposure have been reported. For mice, an LCLo of 370 ppm (1480 mg/m3) for 6 hours exposure has been reported. (RTECS 1995).
In two short-term studies, groups of six adult male rats were exposed by inhalation to furfural.
In the first study (Mishra et al. 1991) rats were exposed at 95 ppm (370 mg/m3) in a single exposure of 1 hour, or at 38 ppm (150 mg/m3) for 1 hour/day, 5 days/week for 7, 15 or 30 days.
In the second study (Gupta et al. 1991) rats were exposed at up to about 220 ppm in single exposures of 1 hour, or at 40 ppm (155 mg/m3) for 1 hour/day, 5 days/week for 7, 15 or 30 days. Six rats were in the control group.
In both studies, furfural exposed animals showed yellowish discoloration of fur, severe irritation of eyes and nose, lachrymation, perinasal and perioral wetness, and mild nasal bleeding. Respiratory difficulty was observed in some animals.
In the first study, the single exposure to 95 ppm resulted in moderate congestion and perivascular oedema in the lungs, these changes were more pronounced after repeated exposure. Changes in some enzyme activities in the lung were observed.
In the second study, an LC50 of 189 ppm was found. Changes in some enzyme activities in the lung were observed.
Oral administration
single exposure
The oral LD50-values in various species indicate that the relative decreasing order of acute oral toxicity of furfural is rat > mouse > guinea pig > rabbit, dog. Oral LD50-values reported are in the range from 65-149 mg/kg in the rat, 333-400 mg/kg in the mouse, 540 mg/kg in the guinea pig, 800 mg/kg in the rabbit, and 950 mg/kg in the dog. (JECFA 1993, Brabec 1994, RTECS 1995).
Rats and mice (5 animals of each sex per group) were administered furfural in corn oil daily by gavage, 5 days/week for 12 doses over 16 days at dose levels of 0, 15, 30, 120, or 240 mg/kg/day for rats and 0, 25, 50, 100, 200, or 400 mg/kg/day for mice.
In rats, low survival rates and laboured breathing were reported at the highest dose level. Animals that received 120 mg/kg were slightly inactive. No compound-related lesions were observed at necropsy.
In mice, no compound-related lesions were noted at necropsy.
(NTP 1990).
Dermal contact
No data have been found.
4.2 Long term toxicity
Inhalation
Syrian golden hamsters (10 animals of each sex per group) were exposed to furfural vapour at concentrations of 0, 77, 448, or 2165 mg/m3 for 6 hours/day, 5 days/week for 13 weeks. At the highest exposure level, the main findings were mild growth retardation, irritation of the eyes and nose, and hyperplastic atrophy of the nasal epithelium. At 448 mg/m3, some mild nasal epithelial degeneration was observed. (Feron et al. 1979 - quoted from JECFA 1993).
Syrian golden hamsters (18 animals of each sex) were exposed to furfural at concentrations of 400 ppm (1550 mg/m3) for 7 hours/day, 5 days/week for 9 weeks, then 300 ppm (1280 mg/m3) during weeks 10-20, and then 250 ppm (970 mg/m3) during weeks 21-52.
Furfural exposure caused yellowish discoloration of the fur, irritation of the nasal mucosa, growth retardation, atrophy and downward growth of sensory cells of the olfactory epithelium, degenerative changes in Bowman's glands, and the occurrence of cyst-like structures in the lamina propria beneath the olfactory epithelium. There was neither evidence of recovery of the nasal changes after a period of six months nor any progression of the lesions. There were no changes in other parts of the respiratory tract or outside the airway system that could be ascribed to furfural. (Feron & Kruysse 1978).
Oral administration
In oral subchronic studies, dose levels of furfural above 50 mg/kg/d were primarily associated with hepatic effects. Mice appeared to be more resistant than rats to the effects of orally administered furfural.
Rats and mice (10 animals of each sex per group) were administered furfural in corn oil daily by gavage, 5 days/week for 13 weeks at dose levels of 0, 11, 22, 45, 90, or 180 mg/kg/day for rats and 0, 75, 150, 300, 600, or 1200 mg/kg/day for mice.
Low survival rates were reported at the highest dose level in rats and the two highest dose levels in mice.
In rats, absolute and relative liver and kidney weights were significantly increased in male rats in the two highest dose levels. The incidences of cytoplasmic vacuolisation of hepatocytes were increased in male rats at all dose levels. There were no compound-related histological lesions in the kidney of male rats.
In mice, a significant increase in relative liver weight was observed in females that received 75, 150, or 300 mg/kg/d and in males that received 300 mg/kg/d. Centrilobular coagulative necrosis of hepatocytes was seen in the liver of 8/10 males and 2/10 females that received 1200 mg/kg, 9/10 males that received 600 mg/kg, 1/10 males that received 300 or 150 mg/kg. Inflammation in the liver was also present when necrosis occurred.
(NTP 1990).
Male rats were fed furfural at a level of 20 ml/kg in the diet (ca. 175 mg/kg/day) for 7 days, 30 ml/kg diet for 7 more days, 40 ml/kg diet (ca. 350 mg/kg/day) from day 15 to 90, and then 40 ml/kg for 5 days/week for a further 30 days. Hepatic cirrhosis was observed in treated animals, and the fibrotic changes were more prominent in animals killed at 120 days than in those killed after 90 days. (Shimizu & Kanisawa 1986 - quoted from IARC 1995 and JECFA 1993).
Dermal contact
No data have been found.
4.3 Reproductive and developmental effects
No data have been found.
4.4 Mutagenic and genotoxic effects
Furfural exhibits an inconsistent pattern of genotoxic activity, being generally negative in bacterial assays but positive in some mammalian cells in vitro.
Furfural did not induce umu c' gene expression in Salmonella typhimurium TA1535/pSK1002 (IARC 1995). Furfural was reported to be mutagenic to Salmonella typhimurium TA100 in the presence and absence of metabolit activation in one study ((Zdzienicka et al. 1978 - quoted from IARC 1995, JECFA 1993 and NTP 1990), but this result was not confirmed in three subsequent studies, which gave equivocal or negative results (IARC 1995). Furfural was reported to be non-mutagenic in Salmonella typhimurium strains G46, TA100, TA1535, C3076, TA1537, D3052, TA1538 and TA98 and in Eschericia coli strains WP2 and WP2 uvrA with a concentration gradient protocol (IARC 1995).
Injection, but not feeding, of furfural to adult Drosophila melanogaster induced sex-linked recessive lethal mutation but not heritable reciprocal translocations (Woodruff et al. 1985 - quoted from IARC 1995, JECFA 1993 and NTP 1990).
Furfural induced gene mutation in mouse lymphoma cells in the absence of metabolic activation (McGregor et al. 1988 - quoted from IARC 1995, JECFA 1993 and NTP 1990).
In in vitro tests, furfural induced sister chromatid exchanges in Chinese hamster ovary cells both with and without exogenous metabolic activation (NTP 1990) and in human lymphocytes without exogenous metabolic activation (Gomez-Arroyo & Souza 1985 - quoted from IARC 1995, JECFA 1993 and from NTP 1990).
In in vivo tests, furfural did not induce sister chromatid exchanges in bone-marrow cells of B6C3F1 male mice injected intraperitoneally with single doses of furfural at doses of 50, 100 or 200 mg/kg b.w. (NTP 1990).
In in vitro tests, furfural induced chromosomal aberrations in Chinese hamster ovary cells both with and without exogenous metabolic activation (NTP 1990, Stich et al. 1981 - quoted from IARC 1995, JECFA 1993 and from NTP 1990) and in Chinese hamster V79 lung cells without exogenous metabolic activation (Nishi et al. 1989 - quoted from IARC 1995).
In in vivo tests, furfural did not induce chromosomal aberrations in bone-marrow cells of B6C3F1 male mice injected intraperitoneally with single doses of furfural at doses of 50, 100 or 200 mg/kg b.w. (NTP 1990).
Furfural reacted with calf thymus DNA in vitro, primarily at AT base pairs, leading to destabilisation of the secondary structure of DNA and to single-strand breaks (Shahabuddin 1991).
4.5 Carcinogenic effects
Rats
Rats (F344/N, 50 animals of each sex per group) were administered furfural in corn oil daily by gavage, 5 days/week for 103 weeks at dose levels of 0, 30 (low), or 60 (high) mg/kg/day. (NTP 1990).
The main non-neoplastic findings were an increased incidence of hepatic centrilobular necrosis in males (3/50, 9/50, 12/50 in controls, low- and high-dose groups, respectively).
No compound-related neoplastic or lesions preneoplastic were observed at any site in female rats. Cholangiocarcinomas were present in two high-dose males rats, and a similar lesion, biliary dysplasia with fibrosis considered to be an early stage in the development of cholangiocarcinoma, was present in two additional high dose male rats. Cholangiocarcinomas are uncommon neoplasms in F344/N rats and have been observed in only 3/2145 corn oil vehicle control male rats in previous NTP 2-year studies.
The NTP conclusions were that there was some evidence of carcinogenicity in male rats, based on the rarity of the biliary pathology. There was no evidence of carcinogenicity in female rats.
Mice
Mice (B6C3F1, 50 animals of each sex per group) were administered furfural in corn oil daily by gavage, 5 days/week for 103 weeks at dose levels of 0, 50 (low), 100 (mid), or 175 (high) mg/kg/day. (NTP 1990).
The main non-neoplastic findings were minimal multifocal chronic inflammation and pigmentation along or immediately below the serosal surface of the liver (pigmentation: 0/50, 0/50, 8/49, 18/50 and 0/50, 0/50, 0/50, 11/50 in controls, low-, mid- and high-dose males and females, respectively; chronic inflammation: 0/50, 0/50, 8/49, 18/50 and 0/50, 0/50, 1/50, 8/50 in controls, low-, mid- and high-dose females, respectively).
Hepatocellular adenomas (both sexes) and carcinomas (males only) occurred with significant positive trends in dosed mice and were significantly increased in high dose groups (175 mg/kg/day).
Forestomach hyperplasia was observed at increased incidences in female mice. Forestomach squamous cell papillomas occurred with at significant positive trend in female mice.
Renal cortical adenomas occurred in 1/49 mid dose and 1/50 high dose males, and a renal cortical carcinoma was seen in 1/50 low dose males.
The NTP conclusions were that there was clear evidence of carcinogenicity in male mice, based on the incidence of hepatocellular adenomas and carcinomas. There was some evidence of carcinogenicity in female mice based on the increased incidence of hepatocellular adenomas.
Samples of mouse liver neoplasms from the NTP-study (NTP 1990) were assessed for transforming gene activity. Liver neoplasms from mice exposed to furfural exhibited a pattern of oncogene activation involving multiple ras alleles, mutations in multiple codons of the same ras allele, and activation of non-ras transforming genes. In contrast, a more restricted pattern of oncogene activation was found in spontaneously occurring liver neoplasms in mice. Of 17 spontaneous liver neoplasms from mice, 15 contained H-ras activated by point mutations in codon 61. The authors concluded that furfural has a direct genotoxic effect in mouse liver.
According to the authors, these novel mutations in ras genes could have resulted from direct genotoxic effects of furfural. The absence of cytotoxic lesions in the liver, based on histopathological examination after 90 days of administration of furfural at the carcinogenic dose argues in favour of direct genotoxic mechanisms. (Reynolds et al., 1987).
Hamsters
Syrian golden hamsters exposed to 0 or 250/400 ppm furfural vapour, 7 hours/day, 5 days/week for 52 weeks. Simultaneously, a proportion of the animals were given either intratracheal instillations of benzo[a]pyrene (BP) or subcutaneous injections of diethylnitrosamine (DENA). There was no evidence of furfural possessing carcinogenic activity. The carcinogenic effect of BP or DENA on the respiratory tract did not appear to be influenced by furfural exposure. For further details, see part 4.2. (Feron & Kruysse 1978).
IARC
Based mainly on the NTP-studies, IARC has concluded that there is limited evidence in experimental animals for the carcinogenicity of furfural.
5. Regulations, limit values
| Ambient air | Denmark (C-value): Furfural is adopted in Main Group 2, class I for organic substances, but without an exact C-value (MST 1990). |
| Drinking water | - |
| Soil | - |
| OELs | Denmark: 2 ppm, 7,9 mg/m3, H (At 1994). |
| Classification | Furfural is classified for acute toxic effects (T;R23/25 - toxic by inhalation and if swallowed) (MM 1993). |
| IARC/WHO | Furfural is not classifiable as to its carcinogenicity to humans (Group 3) (IARC 1995). |
| JECFA/WHO | The Committee considered that no ADI could be allocated to furfural because of the evidence of genotoxicity and carcinogenicity. Direct addition as a flavour was not appropriate, and its use as a solvent should be restricted to situations when alternatives were not available. (JECFA 1993). |
| US-EPA | Oral RfD: 0.003 mg/kg/day, based on a LOAEL of 11 mg/kg/day in a rat oral subchronic study (NTP 1981 - quoted in IRIS 1995, it should be noted that this study is the NTP-study published in 1990) and application of an uncertainty factor of 3000 (IRIS 1995). |
6. Summary
Description
Pure furfural is a colourless, oily liquid with a pungent, aromatic odour resembling that of bitter almonds. Furfural is soluble in water (83 g/l). It has a relatively low volatility.
Environment
Furfural is a volatile component of a wide range of fruits and vegetables. It has been identified as one of the main components of smoke condensates of pine and cottonwood. No data on environmental fate has been found.
Toxicokinetics
Furfural is extensively absorbed and rapidly eliminated in humans after inhalation and in rats after oral administration. In humans, about 78% was retained by the lungs during 8 hours inhalation of the vapour. The pattern of metabolites appears to be qualitatively similar in humans and in rats, involving oxidation of furfural to furanoic acid with subsequent conjugation, primarily with glycine. The conjugate is excreted in the urine. The biological half-life in humans has been estimated to about 2 hours.
Human toxicity
The very sparse human toxicological data indicate that the main effect of furfural is eye and mucous membrane irritation observed after exposure to furfural vapour in concentrations of 20-64 mg/m3. However, no data concerning systemic effects have been found.
Animal toxicity
In animals, furfural has a relatively high degree of toxicity. An LC50-value of 175 ppm (700 mg/m3) for 6 hours exposure has been reported in rat. Oral LD50-values reported are in the range from 65 to 149 mg/kg in the rat.
Repeated exposure of hamsters to furfural vapour by inhalation caused irritation of the eyes and nose, and hyperplastic atrophy of the nasal olfactory epithelium. A NOAEL of 77 mg/m3 (6 hours/day, 5 days/week for 13 weeks) was considered. In oral subchronic and chronic studies, furfural were primarily associated with hepatic effects. For rats, the most sensitive animal, a LOAEL of 11 mg/kg/day (5 days/week) was considered.
Reproductive and developmental effects
No data on reproductive and developmental effects of furfural have been found.
Mutagenic and genotoxic effects
Furfural exhibits an inconsistent pattern of genotoxic activity, being generally negative in bacterial assays but positive in some eukaryotic systems. Neither chromosomal aberrations nor sister chromatid exchanges were observed in bone-marrow cells of mice treated with furfural in vivo. Gene mutation (in a single study), sister chromatid exchanges and chromosomal aberrations were induced in mammalian cells in vitro. Sex-linked recessive lethal mutations were induced in fruit fly.
Carcinogenicity
Furfural has been tested for carcinogenicity by oral administration in rats and mice. In mice, increased incidences (statistically significant in the high dose group of 175 mg/kg/day) of hepatocellular adenomas and carcinomas (males only) were observed. An increased incidence (not statistically significant) of forestomach papillomas were observed in female mice. Male rats had a low incidence of cholangiocarcinomas, which occur rarely.
There was no evidence of furfural possessing carcinogenic or co-carcinogenic activity in hamsters exposed to furfural vapour for 52 weeks.
7. Evaluation
The critical effect of furfural after oral administration to laboratory animals is the hepatotoxicity observed in subchronic and chronic studies. Male rats appeared to be more sensitive to the cytotoxic effects than female rats and mice. In contrast hereto, mice appeared to be more sensitive to the hepatocarcinogenic effects than rats. For mice, the carcinogenic effects were observed at higher dose levels (175 mg/kg/day) than the cytotoxic effects (100 mg/kg/day) and male mice appeared to be more sensitive than female mice.
In hamsters, no indications of carcinogenic effects were observed after exposure to furfural vapours by inhalation for one year. However, that could be due to the relatively short period of exposure.
Furfural exhibits an inconsistent pattern of genotoxicity, being generally negative in bacterial assays, positive in some eukaryotic systems in vitro, and negative in some in vivo tests. Based on these data, no conclusion whether furfural is a genotoxic substance can be drawn. However, the negative response in in vivo tests could indicate that furfural is a non-genotoxic substance.
In conclusion, furfural is considered to be a non-genotoxic substance. The critical effect after oral administration is considered to be hepatotoxicity and after inhalation to be the irritation of eyes and mucous membranes observed in humans.
8. TDI, limit value in air
8.1 TDI
For the estimation of a TDI, the LOAEL of 11 mg/kg b.w. per day (5 days/week for 13 weeks) from the study of rats by NTP (1990) is selected. The LOAEL of 11 mg/kg/day is converted to 7.9 mg/kg/day to adjust for the gavage schedule of 5 days/week.
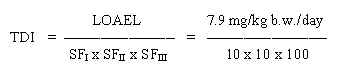
= 0.0008 mg/kg b.w./day
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 100 because of using a LOAEL in stead of a NOAEL, because of uncertainties in establishing a LOAEL from the studies, and because the possibility of genotoxic or carcinogenic effects of furfural cannot fully be excluded.
Allocation
The general population is predominantly exposed to furfural from processed food and beverage products. Therefore, only 10% of the TDI is allocated to ingestion of soil and drinking water and 10% is allocated to exposure from ambient air.
8.2 Limit value in soil
Based on the TDI of 0.0008 mg/kg b.w. per day and assuming a daily ingestion of 0.2 g soil for a child weighing 10 kg (wchild), a limit value is calculated:
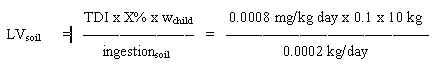
= 4 mg/kg soil
8.3 Limit value in drinking water
Based on the TDI of 0.8 mg/kg b.w. per day and assuming a daily ingestion of 2 litres of drinking water for an adult weighing 70 kg (wadult), a limit value is calculated:
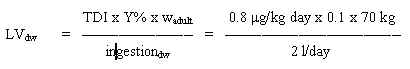
= 2.8 mg/l
8.4 Limit value in air
For the estimation of a limit value in air, a LOAEL of 20 mg/m3 for irritation of eyes and mucous membranes in humans is selected.
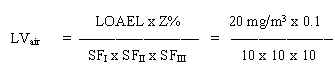
= 0.002 mg/m3
The safety factor SFI is set to 1 as human data are used. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 100 because of using a LOAEL in stead of a NOAEL, because of uncertainties in establishing a LOAEL, and because the possibility of genotoxic or carcinogenic effects of furfural cannot fully be excluded.
9. Quality criteria, C-value
9.1 Quality criteria in soil
A limit value of 4 mg/kg has been calculated based on children’s ingestion of soil. A quality criterion of 4 mg/kg soil is proposed.
Quality criteria
Quality criterion: 4 mg/kg soil.
9.2 Quality criteria in drinking water
A limit value of 2.8 mg/l has been calculated based on intake of drinking water. A quality criterion of 3 mg/l is proposed.
Quality criteria
Quality criterion: 3 µg/l.
9.3 C-value
A limit value of 0.002 mg/m3 has been calculated. For substances having acute or subchronic effects, but for which activity over a certain period of time is necessary before the harmful effect occurs, the C-value is set at the limit value (MST 1990).
Furfural has a low odour threshold (0.31 mg/m3) in air. However, the health based limit value will take into account the discomfort from the odour.
Furfural is adopted in Main Group 2, class I for organic substances, but without an exact C-value (MST 1990). A C-value of 0.002 mg/m3 is proposed. Furfural is moved to Main Group 1 because the possibility of genotoxic or carcinogenic effects of furfural cannot fully be excluded.
C-value
0.002 mg/m3, Main Group 1.
10. References
ACGIH (1991). Furfural. In: TLV's Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 1991-1992. Cincinnati, OH, 694-695.
A&H (1984). Consensus report for furfural. In: Scientific basis for Swedish occupational standards V. Arbete och Hälsa 1984:44. Nordiska expertgruppen för gränsvärdesdokumentation. Arbetarskyddsverket.
Amoore JE and Hautala E (1983). Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J Appl Toxicol 3, 272-290.
At (1994). Grænseværdier for stoffer og materialer. Arbejdstilsynets At-anvisning Nr. 3.1.0.2, juli 1994.
Brabec MJ (1994). Aldehydes and acetals. In: Clayton GD, Clayton FE eds. Patty's Industrial Hygiene and Toxicology, 4th. ed. John Wiley & Sons, Inc, Vol IIA, 283-327.
Feron VJ and Kruysse A (1978). Effects of exposure to furfural vapour in hamsters simultaneously treated with benzo[a]pyrene or diethylnitrosamine. Toxicology 11, 127-144.
Flek J and Sedivec V (1978). The absorption, metabolism and excretion of furfural in man. Int Arch Occup Environ Hlth 41, 159-168.
Gupta GD, Misra A and Agarwal DK (1991). Inhalation toxicity of furfural vapours: an assessment of biochemical response in rat lungs. J Appl Toxicol 11, 343-347.
HSDB (1995). Furfural. In: Hazardous Substances Databank.
IARC (1995). Furfural. In: IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 63, Lyon, 409-429.
IRIS (1995). Furfural. In: Integrated Risk Information System. Database quest, last revised: 01/01/92. US-EPA.
JECFA (1993): Furfural. In: Toxicological evaluation of certain food additives and naturally occurring toxicants. WHO Food Additives Series:30, World Health Organization, Geneva.
Kirk-Othmer (1985). Furfural. In: Concise Encyclopedia of Chemical Technology, John Wiley & Sons, 542-544.
Merck Index (1976). 9th. ed., Rahway, New Jersey, Merck & Co., Inc., 554-555.
Mishra A, Premendra DD, Verma AS, Sinha M, Mishra J, Lal K, Pandya KP and Dutta KK (1991). Pathological and biochemical alterations induced by inhalation of furfural vapor in rat lung. Bull Environ Contam Toxicol 47, 668-674.
MM (1993). The Statutory Order from the Ministry of the Environment no. 830 of October 15, 1993, on the List of Chemical Substances.
MST (1990). Begrænsning af luftforurening fra virksomheder. Vejledning fra Miljøstyrelsen nr. 6 1990.
NTP (1990). Toxicology and carcinogenesis studies of furfural in F344/N rats and B6C3F1 mice. National Toxicology Program, Technical Report Series No 382.
Reynolds SH, Stowers SJ, Patterson RM, Maronpot RR, Aaronson SA and Anderson MW (1987). Activated oncogenes in B6C3F1 mouse liver tumors: Implications for risk assessment. Science 237, 1309-1316.
RTECS (1995). Furfural. In: Registry of Toxic Effects of Chemical Substances. Database quest.
Shahabuddin, Rahman A and Hadi SM (1991). Reaction of furfural and methylfurfural with DNA: Use of single-strand-specific nucleases. Fd Chem Toxic 29, 719-721.
|
[Front page] [Top] |