Pesticides Research No. 124, 2009
Dermal absorption of pesticides - evaluation of variability and prevention
Contents
2 Human skin structure and function
4 Methods to study dermal penetration
5 Biological factors influencing skin absorption
7 Effect of solubility and molecular size on skin penetration
- 7.1 Determining percutaneous penetration rate
- 7.2 Relation between Kρ and logPow
- 7.3 Association between lag-time and solubility
- 7.4 The relation between the lag-time and the molecular weight
- 7.5 Skin deposition and substance solubility
- 7.6 Molecular size in relation to substance solubility and deposition
- 7.7 Exposure in relation to occupational risk assessment
8 Effects of detergents on skin integrity and penetration
9 Concomitant exposure to pesticides and detergents
- 9.1 Approval of commercial products
- 9.2 Penetration profile of commercial products vs. the active ingredient
- 9.3 Mixture of pesticides
- 9.4 Conclusion
10 Penetration of pesticides through slightly damaged skin
- 10.1 OECD guidelines
- 10.2 Work-related skin problems
- 10.3 Trans Epidermal water Loss (TEWL)
- 10.4 Skin-damaging factors
- 10.5 Affecting skin integrity
- 10.6 Conclusion
11 Skin wash and temporary skin deposition
12 Prevention of dermal absorption
- 12.1 Dermal exposure to pesticides
- 12.2 Personal protective gear
- 12.3 Penetration of benzoic acid through gloves
- 12.4 Penetration of pesticides through gloves
- 12.5 Types of gloves
- 12.6 The use and re-use of gloves
13 Conclusions and perspectives
Preface
Through the years great efforts have been made to reduce pulmonary occupational exposure to pesticides. Therefore, the skin has become the primary route of occupational exposure to pesticides. Many factors can change the skin barrier and by that the ability of the pesticides to be absorbed. This is partly due to the physico-chemical characteristics of the active substances as well as the abilities of the detergents used, but also the condition of the skin, i.e. whether the skin is damaged or diseased. In addition, the use of correct gloves can offer protection by limiting and in many cases preventing dermal absorption.
The primary aim of this report is to collect information available in the open literature and information from recent reports published by the Danish Environmental Protection Agency (EPA) in a review including regulatory aspects of the collected information on dermal penetration of pesticides. Thus, the projects have not only scientific aims by revealing and describing facts related to dermal exposure to pesticides, but also aspects related to regulatory and preventive initiatives and guidelines from Danish Governmental Agencies
The results and evaluations are of interests to the workers employed in e.g. greenhouses and agricultures, regulatory agencies as well as departments for occupational and environmental medicine.
The report have been financed by the Danish EPA and the scientific input is to a large extent based on two previously published Danish EPA reports: ’Hudpenetration af pesticider – kombinationseffekter mellem aktiv- og hjælpestoffer’ and ’Penetration af pesticider gennem lettere beskadiget hud’, several peer reviewed articles, and a book chapter all by Jesper Bo Nielsen. The authors have received constructive written comments from an external advisory group (Mari-Ann Flyvholm, Sven Edelfors, Flemming Lander) as well as employees from the Danish EPA (Lærke Ambo Nielsen, Susanne Hougaard, Jørn Kirkegaard).
August 2008.
Rikke Holmgaard
Jesper Bo Nielsen
Sammendrag
Flere projekter er gennem årene blevet udgivet af Miljøstyrelsen med beskrivelse af faktorer af betydning for variationer i hudoptagelsen af bekæmpelsesmidler. For at give en samlet fremstilling af disse projekter og den nyere tilgængelige litteratur er der udarbejdet en detaljeret engelsksproget rapport (Dermal absorption of pesticides – evaluation of variability and prevention). Den rapport er suppleret med en kortere dansk udgave (Dermal absorption af bekæmpelsesmidler – evaluering af årsager til variation samt forebyggelsesmuligheder), der resumerer hovedkonklusionerne fra den engelsksprogede rapport.
Variation i hudoptagelsen efter udsættelse for bekæmpelsesmidler afhænger af personens egen sårbarhed, anvendelse af forebyggelsestiltag, samt af bekæmpelsesmidlernes kemiske egenskaber.
Der er således stor forskel på, hvor godt kemiske stoffer trænger over huden fra forskellige steder på kroppen, ligesom man optager klart større mængder fremmedstoffer over huden, hvis huden er beskadiget med rifter, hudafskrabninger, eksem eller er opblødt efter længere tids vådt arbejde. Det skyldes at hudens beskyttelsesevne i meget høj grad afhænger af det yderste meget tynde lag af huden (stratum corneum). Men der findes heldigvis måder at beskytte sig på. Bruger man for eksempel handsker, vil man være godt beskyttet mod at få noget på hænderne. Her er det imidlertid meget vigtigt at man anvender de rigtige handsker, der passer til de kemiske stoffer, man arbejder med, ligesom engangshandsker kun skal tages på én gang og altså ikke genbruges.. Endvidere vises det, at det virkelig betyder noget at vaske hænder efter at man har haft kontakt til bekæmpelsesmidler.
En række egenskaber ved kemiske stoffer har også betydning for deres evne til at trænge gennem huden. Rapporten dokumenterer, hvorledes store molekyler generelt er længere tid om at passere huden, ligesom rapporten beskriver, hvorledes stofferne opløselighed har stor indflydelse på, hvor hurtigt stofferne passerer huden. Denne viden kan bruges til at opstille simple modeller til forudsigelse af andre fremmedstoffers evne til at trænge gennem huden.
Rapporten har til mål at forbedre og tilgængeliggøre den faglige baggrund for regulatoriske tiltag til forebyggelse af hudoptagelse af bekæmpelsesmidler. Rapporten er tænkt som baggrundsinformation for arbejdsmiljøprofessionelle, faglige organisationer, regulatoriske myndigheder samt Arbejds- og Miljømedicinske klinikker.
Summary
Several projects describing factors of relevance to variations in dermal absorption of pesticides have during recent years been published by the Danish EPA. To summarize these projects and update them with the most recent literature, a detailed report (Dermal absorption of pesticides – evaluation of variability and prevention) was made. This report was supplemented with a shorter Danish version (Dermal absorption af bekæmpelsesmidler – evaluering af årsager til variation samt forebyggelsesmuligheder) focusing on the main conclusions from the English report.
Variability in dermal absorption following exposure to pesticides depends on individual susceptibility, use of preventive measures, and chemical characteristics of the pesticides.
There are large differences between penetration rates of a chemical through skin from different parts of the body. Likewise, dermal absorption of chemicals is significantly enhanced through skin compromised by minor scrapes, atopic dermatitis, eczema, or by hydrated skin due to wet work. The reason is that protection against dermal absorption of chemicals mainly depends on the condition of the upper very thin layer on the skin (stratum corneum). Fortunately, personal protective equipment such as gloves exists that will prevent or reduce dermal exposure of the hands. It is, however, of immense importance to use the type of gloves suitable for the pesticide in question, and not use disposable gloves more than once. Further, evidence is presented that hand wash following dermal exposure to pesticides significantly reduces absorption.
Different chemical characteristics of pesticides affect their ability to penetrate human skin. This report presents evidence that large molecules (high molecular weight) generally require more time to be absorbed through the skin. Likewise, solubility characteristics of the pesticides will affect penetration rates. This knowledge may be used for establishing mathematic models that can be used to predict dermal penetration characteristics of other chemicals.
This report is aimed at improving the scientific background and the accessibility of knowledge on regulatory measures to prevent or reduce dermal absorption of pesticides. The report is intended to be used as background information by occupational health professionals, labour organizations, and regulatory agencies.
1 Introduction
Pesticides are among the few substances dispersed into our environment with the intent to harm biological systems. The selectivity of pesticides varies and many of the toxicological endpoints that pesticides target also make humans a potential target.
Occupational and household exposure to pesticides occurs during mixing and spraying and in greenhouses during re-entry activities as the plants are handled right after the pesticide treatment. The dermal absorption is known to be a process of passive diffusion that can be divided into several different steps. Recent studies have shown that the rate of absorption is related to the solubility of the pesticide, the presence of detergents and the integrity of the skin barrier (Brand & Mueller, 2002;Nielsen JB, 2004;Nielsen et al., 2004). So far the existing procedures for approval of pesticides by the Danish Environmental Protection Agency have not taken the changes of detergent in already approved commercial products into account, nor have they focused on the deposition of pesticides in the skin or the integrity of the skin barrier.
The overall aim of this report is not to uncover new effects related to the passage of pesticides through skin but to clarify, describe, and summarize present knowledge on dermal penetration of pesticides and to discuss potential consequences for regulatory guidelines implemented and used by regulatory agencies.
The specific purpose of this report is to:
- Describe an interval in relation to physico-chemical characteristics, where the highest dermal absorption would be expected.
- Describe the importance of temporary deposition in the skin (reservoir effect) in relation to delayed absorption after end of exposure.
- Discuss whether washing the skin after exposure might remove part of the pesticide deposited on or within the upper stratum corneum, and whether regulators should continue seeing this fraction as de facto absorbed.
- Assess potential kinetic interactions in the absorption of mixtures of pesticides.
- Assess the influence of specific detergents used in formulation of commercial products on dermal penetration.
- Assess the importance of slightly damaged skin for dermal absorption as well as temporary deposition in the skin.
- Assess the effect of personal protective equipment (PPE), in the form of different types of gloves.
The understanding of dermal absorption of pesticides is still limited and publicly available information has mainly been focusing on specific substances, e.g. neat chemicals, and their ability for penetration. In real life the sales products that people are exposed to are mixed products which besides the pesticide also contain different detergents, stabilizers or solubilizers. When making a risk profile of the pesticides it is therefore important not only to assess the toxicity of the active substance both also evaluate the toxicity of the detergents and their effect as mediators on the absorption of other substances. A mediator may enhance the dermal absorption and thereby enhance the bioavailability of the substances (Sartorelli et al., 1997), but it may also directly affect the skin barrier (Treffel P & Gabrad B, 1996;TupkerRA, 1990). As a consequence, EU guidelines for evaluation of dermal penetration and dermal toxicity (EC Directive 91/414) prescribe testing of the active substance as well as the sales product.
Percutaneous penetration of pesticides has been studied in vivo in animals and in vitro by the use of animal or human skin samples. Rodent skin has been shown to overestimate the penetration rate of most topically applied compounds (OECD, 2000;van de Sandt et al., 2004). The present report will whenever available and valid data exist rely on data from studies with human skin based on experimental models described in the most recent OECD guidelines, which have generally had a reasonable good correlation with human in vivo studies (OECD, 2000;Ramsey et al., 1994). Skin thickness will affect the experimental results (van de Sandt et al., 2004;Wilkinson et al., 2006), and prolonged lag-times might be expected in experiments using full-thickness skin. The most reliable model generating most credible data is not obvious, and this is probably one of the reasons why OECD accepts the different experimental approaches in their guidelines. A recent inter-laboratory comparison of experimental models on percutaneous penetration involving nine European laboratories demonstrated good agreement between data on selected model compounds obtained in the different laboratories, given that comparable experimental procedures were used (van de Sandt et al., 2004).
1.1 Relevance
The primary aim of this report is to assist regulators on use of pesticides in recognizing risks when using these products and to present relevant information on preventive measures related to substitution to less harmful products and use of personal protective equipments. Currently most of this information is available as scientific reports unattainable from general library search systems or as separate papers published internationally. The present report will collect information available in the open literature and information from recent reports published by the Danish EPA in a review including regulatory aspects of the collected information. Thus, the projects have not only scientific aims by revealing and describing facts related to dermal exposure to pesticides, dermal penetration, but also aspects in regulation and prevention in relation to guidelines from Danish Governmental Agencies.
2 Human skin structure and function
The skin is a complicated human organ (Figure 1), which is continuously exposed to chemicals, mechanical injury, micro-organisms, UV light, temperature fluctuations and water loss. The most important function of this organ is to minimize unintended water loss and maintain homeostasis and to act as a barrier against these exposures. As the largest single organ of the body, the skin accounts for more than 6% of the body mass; on average about 5 kg covering 1.8m² depending on height and weight (Roberts MS & Walters KA, 1998b;Thestrup-Pedersen K et al., 1993). The cutaneous blood circulation comprises 5-10% of the total cardiac output (Johnson et al., 1986).
The skin is a heterogeneous organ, holding a number of layers as well as appendages – hair follicles, sebaceous glands and sweat glands. Different body regions show different skin thickness and composition of the stratum corneum. The skin can be divided into an outer region - the upper epidermis and an inner region - the lower more vascular dermis. The viable epidermis can metabolize chemicals that infiltrate the stratum corneum. The dermis offers physiological support for the avascular epidermis and is the source of nutrition.
2.1 Epidermis
The epidermis can be divided into four layers (from inner to outer layer):
2.1.1 Stratum basale (germinativum)
Following the life circle of the keratinocytes they start out as a single layer of columnar basal cells attached to the basement membrane by hemidesmosomes creating the stratum basale (Roberts MS & Walters KA, 1998b). They consist of metabolically active cells, which continuously proliferate and undergo mitosis, causing the older cells to move out towards the surface.
2.1.2 Stratum spinosum
Stratum spinosum lies on top of the stratum basale and is also known as the prickle layer. These cells adjacent to the basal layer generate lamellar granules that soon after fuse with the cell membrane to release the neutral lipids thought to generate a barrier to penetration through the epidermis. This part of the epidermis consists of many layers of irregular cells connected to the surrounding cells by desmosomes. As the cell layers move outwards they flatten, and the granules reflect the border between this stratum and the overlying stratum granulosum.
2.1.3 Stratum granulosum
The cells in stratum granulosum are even more flattened than in the previous layer. They contain an increasing amount of keratin as further keratin differentiation occurs in this layer. The most characteristic elements are the intracellular granules. They hold many different components that all together play an important role in the keratinisation process of the cells. Enzymes degrade the viable cell components such as nuclei and intracytoplasmatic organelles. The cells in the stratum granulosum also contain large amounts of filaggrin, a protein thought to serve in bundling keratin. Filaggrin is an element in the keratinisation process which together with lipids helps to create a protective barrier against penetrating substances. Mutations in the filaggrin gene have proved to create an impaired barrier function as it gives a varying degree of abnormal skin conditions (Palmer et al., 2006). This will be described in the section dealing with the barrier function.
2.1.4 Stratum corneum
Stratum corneum, also known as the horny layer, develops from immature, columnar, keratinocytes that, as they move from the basal layer and by that the source of nutrition to the stratum corneum, become flat, keratin-filled and dead cells. On the way to the surface, they loose the nucleus and the capacity for metabolic activity. From the body surface the dead cells are constantly shedded and therefore the skin barrier is continuously renewed. The total cell turnover of epidermal cells is between 17 and 71 days depending on the anatomical site (Maibach H & Patrick E, 2001). The stratum corneum provides almost all mechanical strength to the epidermis.
The stratum corneum varies in thickness (10-600mm) depending on the area of the body and the physical interaction that the skin is exposed to in daily life. Plantar and palmar callus can be 400-600mm thick compared to 10-20 mm for the back, arms, legs and abdomen (Scheuplein & Blank, 1971).
The stratum corneum consists of several flattened, hexagonal and cornified cells stacked on top of each other, usually 15–20 cells thick. It is a heterogeneous structure containing about 40% protein (mainly keratin, a disulfide cross-linked linear polypeptide), 40% water (depending on humidity) and about 15 to 20% lipids (principally triglycerides, free fatty acids, cholesterol, and phospholipids) (Michales AS et al., 1975). The lipid is concentrated in the extracellular phase and the protein in the intra- and extracellular phase. Stratum corneum’s protein-rich corneocytes embedded in a matrix of ceramides, cholesterol, and fatty acids, and smaller amounts of cholesterol sulphate, glucosylceramides and phospholipids were described by Elias (1983) and Bouwstra (2006) as the “Brick and mortar” model (Elias, 1983;Bouwstra & Ponec, 2006) and later by Forslind (1997) in a more detailed model “the domain mosaic model” (Forslind et al., 1997). Because of the contents of lipids it has a low permeability to many agents and a protective function for the individual. The bricks (keratinocytes) act as a hydrophilic membrane and are almost impenetrable to water and by that a regulating mechanism to limit transepidermal water loss. The mortar (lipids) acts as a regulating mechanism for hydrophobic penetration.
A previous study has indicated that a too high as well as a too low lipophilicity limits the skin penetration of a substance (Nielsen JB, 2004), but further research needs to be done to determine an interval in relation to lipophilicity where the most efficient absorption could be expected. This information will potentially allow an improved prediction of the ability of new chemicals to penetrate the skin.
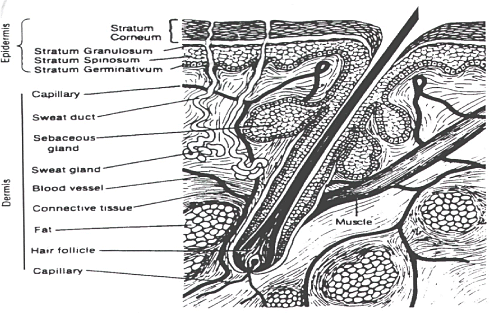
Figure 1: Skin structure.
2.2 Dermis
The epidermis rests on the much thicker dermis (2000mm), which again lies above the subcutaneous fat. Dermis consists of collagenous fibres (70%), providing a scaffold of support and cushioning, and elastic connective tissue, providing elasticity, in a semigel matrix of mucopolysaccharides. The dermis also embeds mastcells (part of the immune system), melanocytes (produce pigment) and the fibroblasts (produce the connective tissue). Moreover, the dermis also carries the blood, nerve and lymph supply. This vascular network of microcirculation supports the skin with nutritients and acts as a heat regulator besides absorbing penetrated chemicals and transporting them to the systemic circulation (Guy et al., 1987). The nerves respond to pressure and temperature. The lymphatic system is an important component of the skin in regulating its interstitial pressure, mobilization of defence mechanism, and in waste removal (Roberts MS & Walters KA, 1998a). While blood flow establishes the clearance of small solutes such as water and other small particles, lymphatic flow is an important determinant in the dermal removal of larger solutes (Cross & Roberts, 1993).
2.3 Skin appendages
The skin contains different appendages such as hair follicles with its sebaceous glands, eccrine glands, apocrine glands and nails. Each has different functions that provide the skin with its many abilities to protect the human body.
The hair is a keratinised structure that acts as protection and is distributed on most of the body. The hair cycle is controlled by temperature, light, nutrition and hormones. The hair follicles are rooted in the dermis, sometimes extending into the hypodermis.
The sebaceous glands have a protective function. They secrete sebum, which acts as lubricant to waterproof the skin and prevent it from drying. Androgenic hormones stimulate the sebaceous glands.
The eccrine glands are sweat glands that are found all over the body with a majority on the soles, palms and axillae. The sympatic nerves activate them and the function is to regulate body temperature.
The apocrine glands are found in the axillae, the nipples and the anogenital area and secrete proteins, lipids and lipoproteins. They are also sweat glands, but with a different function and morphology than the eccrine glands. Both types of glands are activated by the sympathetic nerve system (Roberts MS & Walters KA, 1998b).
3 Skin penetration
Skin penetration is of great importance, clinically, occupationally as well as environmentally. Many people have been and are still unintentionally exposed to toxic substances either at work or at home, such as exposure to dust, pesticides and detergents. Skin absorption (if not massive) is difficult to quantify and is therefore rarely proven to be a significant route of entry even when people experience problems after years of being exposed dermally to harmful substances.
It is therefore of great value that occupational health agencies focus on these problems and try to promote and enforce safe production methods and working conditions.
3.1 Absorption mechanism
Chemicals penetrate the stratum corneum by passive diffusion – whereas active transport plays a limited role (Scheuplein & Blank, 1971). Chemicals pass the upper skin structures into the viable epidermis and continue passively through to the dermis – the dermal-epidermal junction - where the blood vessels will transport it to the systemic circulation.
A pharmacokinetic model (Figure 2) has been made to describe the absorption through the skin. The model is linear and describes the percutaneuos absorption using three first-order rate constants.

Figure 2. Model of the absorption across the skin barrier.
k1 describes the diffusion across the stratum corneum. k2 is the transport to the viable epidermis.
k3 reflects the affinity of the penetrant for the stratum corneum versus the viable epidermis.
The figure also illustrates the potential for an accumulation of the penetrant in the stratum corneum.
The k3/k2 ratio provides the “effective partition coefficient” of the penetrant between the two layers (Guy et al., 1985). To what extent the model should also include an arrow from stratum corneum to the skin surface due to cell shedding or skin wash will be discussed in Chapter 11.
The permeability coefficient kρ depends on the solute size, lipophilicity and the diffusion path length. Even though Fick’s law describes the thickness of the skin as having influence on the penetration and Scheuplein and Blank (1971) indicate the skin thickness as being the controlling factor in skin penetration, Elias et al. (1981) were unable to determine any association between absorption and neither the thickness nor the number of cell layers in the skin. There were indications that the intercellular lipids were important factors in the regulation of epidermal permeability (Elias, 1981;Elias, 1983). Later works also show that penetration depends more on the lipid composition than on the skin thickness. Even though different sites have the same thickness or lipid content it does not mean that they by definition have the same penetration rate, all due to day-to-day structure variations (Bronaugh & Maibach, 1985).
For a substance to be transdermally absorbed some key events must take place:
- The substance interacts with the stratum corneum.
- Diffusion of the substance through the stratum corneum.
- Crossing from the lipophilic stratum corneum to the more aqueous viable epidermis.
- Continuing from the avascular epidermis to the highly perfused dermal tissue.
- Uptake through the microcirculation to the systemic circulation
(Clark NWE, 1992;Guy et al., 1987;Kao et al., 1988).
When the substance has to pass the stratum corneum it generally has two pathways in humans: a) Transcellular and b) Intercellular (Figure 3), the major route being the intercellular pathway between the corneocytes, implying that stratum corneum lipids play an important role in the skin barrier function (Cnubben et al., 2002;Elias, 1981;Michales AS et al., 1975). However, for very lipophilic and large molecules (and some electrolytes) the appendages and other diffusion shunts may also play an important role (Kao et al., 1988).
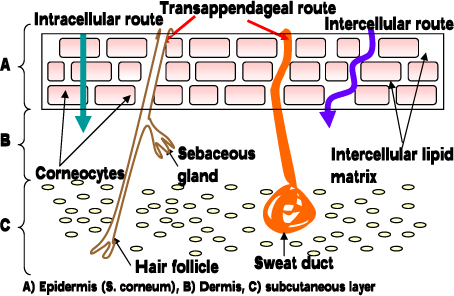
Figure 3. Pathways through the skin.
3.2 Absorption kinetics
A skin penetrating substance first has to pass the avascular and lipophilic structure (stratum corneum) and continue through a more aqueous layer (lower epidermis and dermis) to the blood vessels. The permeability coefficient of the substance increases as its lipophilicity increases (Roy & Flynn, 1989). A lipophilic compound will easily cross the stratum corneum but the penetration rate will decrease as it reaches the hydrophilic epidermis and the diffusion of the substance will slow down. The consequence is a temporary deposition within the skin. This process is called a reservoir effect and will be described and discussed later. Substances soluble in the lipophilic layer as well as in the more aqueous structures and at the same time, having a small molecular size have the best permeability through the skin barrier (Guy et al., 1987). Electrolytes on the other hand are difficult to absorb when they are applied in aqueous solutions. Ions create a field of stable hydration that increases the size of the diffusing component (Grandjean P, 1990).
3.2.1 Fick’s first law
Fick’s first law of diffusion only applies under very specific conditions. It will, however, give a good approximation of flux rates related to dermal penetration (Grandjean P, 1990).
JSS = kP * ΔC
JSS = flux of penetrant molecule under steady-state conditions (absorption rate). k? =permeability coefficient of the penetrant through the membrane and ΔC = concentration gradient across the membrane.
The determination of the permeability coefficient (Figure 4) may also be calculated from:
kP = JSS /A * ΔC = K * D /h
A = application area (Franz cell opening). K = the skin/vehicle partition coefficient of a solution. D = apparent diffusion coefficient. h = the diffusional path length. Since all but KP are known parameters, KP may be calculated.
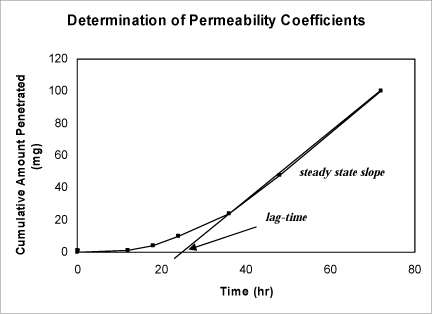
Figure 4. Graph showing how to determine steady state flux (used to calculate the permeability coefficient) and lag-time.
The steady-state flux, JSS,is the slope of the linear part of the graph of the cumulative amount penetrated as a function of time. The lag time is the time intercept of the linear portion of the graph.
3.2.2 Prediction of permeation
Over the years many calculations have been made to predict the permeability coefficient by a simple model based on the molecular weight MW of the chemical. The estimation of kp has been based on data on mainly hydrocarbons, since for hydrocarbons, the ratio of molecular weight to molecular volume is nearly constant and therefore a kp estimate based on molecular weight is as good as one based on molecular volume and it has shown that there is evidence that maximal flux and penetration decrease exponentially with molecular weight (Kasting GB et al., 1987;Potts & Guy, 1992). In 1992 Kasting developed an equation based on the theory that solute transport could follow a polar pathway with a permeability coefficient kp,polar as well as follow an intercellular lipid pathway with a permeability coefficient kp,lipid, even though the existence of a polar pathway still remains controversial. For lipophilic solutes, an aqueous layer is likely to be permeable at the stratum corneum epidermis interface with a kp,aqueous. Hence,
kp = [1/(kp,lipid +kp,polar) + 1/kp,aqueous]-1 (Kasting GB et al., 1992)
Kasting et al considered the range for kp,polar to be 10-5 to 10-6 (300/MW)½ cm/h and kp,aqueous ~ 0.15 x (300/MW)½, the second being comparable to permeability of solutes through delipidized stratum corneum.
For most solutes:
kp,lipid >> kp,polar and kp,lipid << kp,aqueous,
so that kp ~ kp,lipid. (Kasting GB et al., 1992)
Usually, solute lipophilicity favours skin permeability with the diffusivity of the solute being higher for solutes with less affinity to bind to hydrogen.
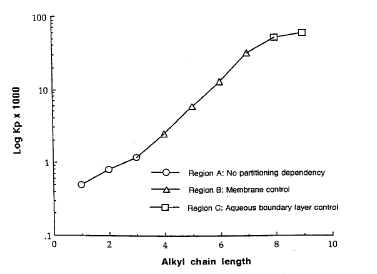
Figure 5. Consequence of the substance lipophilicity on the rate of skin penetration. (Figure from Roberts and Walters)(Roberts MS & Walters KA, 1998a): Average permeability results for aqueous alcohol solutions through the stratum corneum of human skin as a function of alcohol chain length (Scheuplein and blank 1973).
The equation from Kasting et al determinates the kp,lipidby using the molecular weight and the octanol-water partition coefficient:
log kp,lipid= log koct – (0.018/2.303)*MW – 2.87 (Kasting GB et al., 1992)
In 1992 Potts and Guy came up with a similar equation assuming a single pathway through a multiple regression of 97 solutes defined by Flynn (Flynn GL, 1990):
log kp = 0.71*log koct – 0.0061*MW – 2.72 (Potts & Guy, 1992)
For dense compounds it was found that the molecular weight was a larger relative to molecular volume than for hydrocarbons and the kp value calculated from an equation using molecular weight showed underestimation of the kp(Vecchia BE & Bunge AL, 2003).
The major limitations of models predicting the permeability coefficient are that the models are based on permeation from simple aqueous solutions where no physiological factors or formulation effects are considered.
3.2.3 Octanol-water partition coefficients
As mentioned above, the physicochemical constant log koct is used to describe the lipophilicity of the penetrant. koct is the octanol-water partition coefficient, defined as ratio of the equilibrium concentrations of the penetrant in a two-phase system consisting of two immiscible solvents, (octanol and water).
koct = Coctanol / Cwater
The partition coefficient is given as its logarithm to base ten, meaning that a high log koct value indicates a high lipophilicity (Clark NWE, 1992) and by that a qualitative indicator of penetration (Potts & Guy, 1992). Substances with high lipophilicity tend to remain in a reservoir in the lipophilic part of the skin. It is a matter of balance. A large amount of absorption is association with log koctvalues of 1 to 2 and decreasing considerably when exceeding 3.5 (ECETOC, 1993)
3.2.4 Lag-time
Lag-time is the time from the penetrant is applied to the skin surface (the start of the exposure) until it is possible to detect the substance on the other side of the skin. The importance of knowing the lag-time is illustrated in Figure 6 which shows the penetration of a test-substance in two different formulations (mixed with different detergents). It shows that the total amount of substance penetrated is the same, but the lag-time and flux are different.
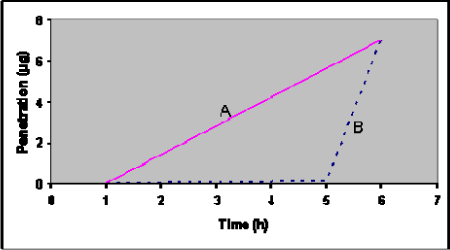
Figure 6: Theoretical penetration curves for two chemicals (A and B) with identical total penetration after 6 hours, but different lag-time and flux.
Some substances penetrate the skin slower than expected due to binding. The skin may act as a reservoir (see below) because of some substances’ physicochemical properties (Vickers, 1972). The substance will deposit in the epidermis thereby delaying the systemic effect. This phenomenon also controls the lag time. Lipid solubility is a predictor of the drug’s solubility in the skin, and with greater lipid solubility a larger deposition, longer lag time and prolonged elimination behaviour will be expected (Guy et al., 1985;Knepp et al., 1987;Plezia et al., 1989;Roy & Flynn, 1989;Roy et al., 1994;Scheuplein & Blank, 1971).
Lack of knowledge about the lag-time of different pesticides makes assessment of exposure and risk difficult. There is a risk of underestimation or completely overlooking an ongoing or recent exposure. A lag-time of 4 hours does not mean that the pesticide is still on the skin surface after 4 hours before the penetration starts, but reflects the fact that penetration takes time and depends on the penetrant. The passive absorption is a multi-step process. Initially there is absorption from the often hydrophilic donor phase on the skin surface. This is accompanied by diffusion through the skin with a temporary deposition (reservoir effect) and at the end penetration to the receptor. The passive diffusion from the often hydrophilic donor and into the skin is favoured by the lipophilicity of the substance (Nielsen et al., 2004).
3.2.5 Reservoir effect
When a substance stays and accumulates in the skin instead of passing directly through to the bloodstream it is described as a reservoir. A reservoir can be present in the stratum corneum, the viable epidermis or in the dermis (Roberts et al., 2004). The substance staying in the reservoir will often be released with a certain “delay” to the blood stream or maybe back to the skin surface, a possible option that will be discussed later in Chapter 11. The absorption of the substance into the blood stream continues from the application site at a gradually declining pace, giving the appearance of prolonged elimination (Lee FW, 2000) (Cnubben et al., 2002). The reservoir effect can be induced by occlusion with plastic film, but it will depend on the substance, the temperature of the skin and the humidity (Vickers, 1972). When studying the reservoir effect the flow-through system is useful (see below). Studies have shown that absorption from washed skin after end of application in an in vivo study on rats continued for almost all pesticides (Zendzian, 2003). Yet another study showed the importance of tracking down chemicals remaining in the skin. In 2004, Yourick et al. concluded in a flow-through study investigating the penetration of three substances that two of the substances made reservoir in the epidermis, and by that the substance should not be considered as absorbed material. Data from the last substance, however, indicated that the substance was spread throughout the epidermis and dermis and therefore could not be excluded from the absorbed dose (IPCS, 2006). In the present studies we will try to evaluate the effect of washing an exposed skin area to avoid absorption of a pesticide.
4 Methods to study dermal penetration
During the last decade much attention related to percutaneous penetration has been directed towards standardization and validation of experimental models. In vitro as well as in vivo methods exist, each with their own set of advantages and disadvantages. Which method to use will depend on the research question to be answered, which is probably also one of the reasons why the present OECD guideline for studies on percutaneous penetration accepts several methods. Equally important to the researcher generating experimental data as to the user of this data (e.g. governmental agencies) is a clear appreciation of the pros and cons that each method has. This chapter will describe some of the frequently used experimental methods and their different advantages and limitations.
4.1 In vitro technique
Standardization and validation of different in vitro models have been described by the Percutaneous Penetration Subgroup of EC Dermal Exposure Network (Sartorelli et al., 2000). A recent inter-laboratory comparison of experimental models on percutaneous penetration involving nine European laboratories demonstrated good agreement between data on selected model compounds obtained in the different laboratories, given that comparable experimental procedures were used (van de Sandt et al., 2004).
In vitro methods are used in laboratories all over the world with the intention to assess the penetration characteristics of specific substances. A range of different designs have been developed with the general aim to measure the penetration of agents through the skin membrane into a fluid reservoir. As previously described, several factors influence the permeation of a substance such as solubility, molecular weight and size, penetrant-skin binding, barrier function etc.
In 2004 OECD issued a guideline for testing chemicals by in vitro methods. The standard principles were described for the use of the static diffusion cell on the flow-through system. The test substance is in both methods applied to the surface of the skin which separates the donor and the receptor chambers. The amount of penetrated substance is measured in the receptor fluid as a function of time. Experimental approaches include infinite as well as finite dosing and may also include setups where test substance remains on the skin for a specified time under specified conditions, before removal by an appropriate cleansing procedure. It is important to keep the receptor fluid (and sometimes the donor substance) homogeneous by stirring (OECD, 2004).
4.1.1 Static diffusion cells
In 1975 Franz developed a static diffusion cell which is now one of the most commonly used in vitro systems in the research of skin penetration. The system has a simple design and is inexpensive to use (Figure 7). Human as well as animal skin can be mounted on the metal grid which divides the donor chamber and the receptor chamber. The skin is set placing the dermis in contact with the receptor fluid below. The skin can be either full-thickness or split-thickness skin. The skin thickness will affect the experimental results (van de Sandt et al., 2004) as elaborated under Flow-through system. The receptor chamber of the cell is placed in circulation water in a water bath with a temperature of 37 ºC keeping the temperature at the skin surface at 32° to imitate a real life skin condition as much as possible. The receptor fluid is kept homogenous in concentration and in temperature by a magnetic stirring bar. The fluid in the receptor chamber is manually sampled at predefined time intervals. Any type and any amount of vehicle (that will fit into the donor chamber) may be applied to the skin.
Franz showed an excellent correlation between in vitro and in vivo studies (Franz, 1975).
When testing different substances it is important to be aware of the solubility of the substance. The solubility of a substance influences the sink capacity and is therefore of great importance when it comes to choosing the right sampling frequency and receptor chamber dimension. The size of the receptor chamber determines when the receptor fluid achieves a certain degree of saturation (Brain KR et al., 1998).
The barrier integrity of the skin can be evaluated by capacitance measurement. This value indicates the ability of the skin to separate electrical charge.
Skin samples with a high capacitance are unable to act as capacitors, which means that the skin is damaged. The measurements are carried out at the beginning and at the end of the study to give an accurate evaluation of the skin barrier.

Figure 7: Static diffusion cell.
4.1.2 Flow-through system
Another in vitro model is the flow-through system (Figure 8 and 9). This is a system consisting of multiple cells. The system is developed by Bronaugh and Stewart in 1985 and is excellent for determining the reservoir effect of the skin (Bronaugh & Stewart, 1985a). The flow-through cells can - as well as the static cells - be mounted with animal or human full- or split-thickness skin, which will generate skin barriers of different thickness and as in the static cells the skin thickness will affect the experimental results (van de Sandt et al., 2004). Prolonged lag-times might be expected in experiments using full-thickness skin. The type of skin preparation generating the most valid data is not obvious and probably one of the reasons why OECD accepts the different experimental approaches in their guidelines (OECD, 2000).
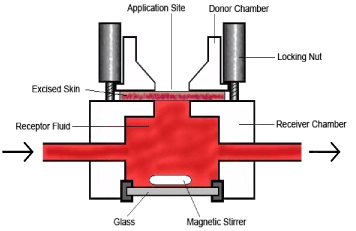
Figure 8: Flow-through cell (Use of picture permitted by Dr. Wilkinson SC, University of Newcastle).
The receptor fluid is in the flow-through cells continuously replaced and collected every hour to imitate an in vivo situation where the blood circulation removes the transdermal penetration substances. This has an additional benefit when dealing with substances with low solubility in the receptor medium and the sink conditions are maximized as the fluid is continually replaced (Brain KR et al., 1998). The donor chamber is, however, very small which gives a small application area, and it is also important to be aware what the system lag-time does to the volume of the receptor chamber and the outlet tubing, which is highly expressed when using a low flow rate. Depending on the amount of cells used in the study, the amount of connecting outlet tubes leading the receptor fluid from the cells to the collecting vials can be quite confusing (see picture below). The mechanical movement, when changing collection vials e.g. every ½ hr., has a tendency to disconnect the tubes.
Figure 9: Flow-through cells (Use of picture permitted by Dr. Wilkinson SC, University of Newcastle).
4.1.3 Advantages/disadvantages
In general the in vitro models have the advantage of avoiding almost all ethical aspect. Since many percutaneous penetration studies would be hazardous to carry out in vivo, e.g. studying chemical warfare agents, the in vitro models meet these risks (Vallet et al., 2007). The static diffusion cells have some advantages compared to the flow-through system, simply due to the much simpler design in the static system. The static diffusion cells have except from the magnetic stirrer no technical features, and this therefore eliminates many of the technical problems that may occur when using the flow-through system. The costs of the static diffusion cells are lower than those of the flow-through system and the static diffusion cells have a larger area of absorption which makes the absorption indicator better as well as the mass balance assessment. The flow-through system, however, provides an environment similar to real physiological conditions by the continuous replacement of receptor fluid (Bronaugh, 2004a;Bronaugh, 2004b) resembling the systemic uptake of the drugs/chemicals in the blood vessels.
Many comparative studies with no difference in skin penetration measurements between the two cell types have been carried out (Bronaugh & Maibach, 1985;Bronaugh & Stewart, 1985a;Chilcott et al., 2005;van de Sandt et al., 2004).
The models are both originally described by OECD guideline 428 (OECD, 2000) for experiments with skin penetration.
4.2 In vivo technique
In vivo technique is based on a physiologically and metabolically intact system. There are two kinds of in vivo studies: 1) animal studies and 2) human studies.
The most frequently used animal is the rat even though it is well known that rat studies generally overestimate human skin absorption (ECETOC, 1993). Other animals demonstrate a better agreement with human absorption, but the costs of these are considerably higher. Human studies are preferable in order to avoid extrapolation between species, but the ethical issues can be extensive.
In 2004 OECD issued a guideline for testing chemicals by in vivo methods. The standard principals described were application of the test substance to the skin in proper form and time, taking samples of different body fluids, excreta or tissue at specific intervals, and quantifying the test substance in the samples or the metabolite in the samples by an appropriately sensitive analytical method.
4.2.1 Traditional in vivo technique
The gold standard is of cause where the test substance is applied to the skin of healthy humans and blood and/or urine is collected and analysed. The amount of test substance measured in the blood and/or urine gives a good indication of the amount of substance absorbed through the skin into the systemic circulation. This in vivo technique has been used before all other techniques were ever considered and is still used where there is no adverse risk to the volunteers participating (Benech-Kieffer et al., 2003;Hueber-Becker et al., 2004;Lammers JHCM et al., 2005;Nohynek et al., 2004;Nohynek et al., 2006;Hueber-Becker et al., 2007). Often the test of a substance is intended to disclose different unknown characteristics of the substance signifying that the knowledge of the substance is limited or incomplete and therefore the risk assessment may not be completely explained. This gives rise to ethical concern and limits the use of healthy volunteers.
4.2.2 Microdialysis
Microdialysis is a technique used in the clinic as well as in research for sampling of endogenous and exogenous substances in the extracellular space in the living tissue. Microdialysis is so far the only technique that provides information from the extracellular space, and it is therefore of great importance in the investigation of pharmacological and biochemical procedures in these tissues. Several results in drug discovery and development are established by measuring serum concentrations of different molecules, even though most drugs exert their effects in the tissues and not in the blood stream. Thus, data on pharmacokinetics at the target site are important, just as determination of pharmacodynamic effects in relation to tissue drug concentrations in the target tissue is a more precise approach to describe exposure effects (Chaurasia et al., 2007). Microdialysis is currently the most essential tool to estimate active drug profiles at the target site and for providing pharmacokinetic and pharmacodynamic information.
Microdialysis is an in vivo sampling technique that can be used to measure endogenous and exogenous substances in the extracellular space in living tissue, e.g. the skin. The method was originally developed in neuropharmacological sciences (Ungerstedt U, 1984), and is now widely used in animal as well as human models to measure substances in different target tissues (Chaurasia, 1999;Kreilgaard, 2002;Lonnroth et al., 1987;Muller, 2002;Stahl et al., 2002), and for investigation of skin absorption. The first report concerning cutaneous microdialysis was published in 1991 (Anderson et al., 1991). This technique involves the insertion of a probe into the dermis (Figure 10).
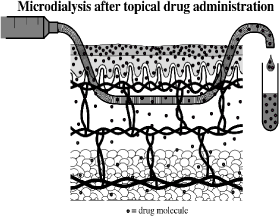
Figure 10: Microdialysis. Probe inserted in the dermis. (Benfeldt & Serup, 1999)
The in vitro techniques have limitations when it comes to studying metabolic, pharmacological and biochemical aspects because of the missing perfusion of the skin. Methods used in vivo – as tape stripping described below – are also inadequate when it comes to looking at metabolism and biochemical characteristics. Here microdialysis has an advantage as the technique makes it possible to examine not only what goes on in the upper layer of the skin but also deeper down in the dermis.
Principals in microdialysis are to imitate the function of a small blood vessel in the dermis. A test substance applied to the skin will penetrate the skin surface to the dermis where the artificial blood vessel/probe is placed. The probes consist of a semi-permeable structure which allows molecules to pass into the perfusate inside the probe by passive diffuse. The molecules follow a concentration gradient across the probe membrane as the perfusate inside the probe passes through, driven at a constant and very accurate pace by a pump. A partial equilibration of molecules across the membrane occurs. The perfusate – now called the dialysate - leaves the probe, holds the test substance and is collected in small vials for analysis. This technique has been used in human volunteers as well as in animals (Benfeldt & Serup, 1999;Benfeldt et al., 2007;Groth, 1996).
4.2.3 Tape stripping
Tape stripping is a well known in vivo technique but can also be used in vitro. A test substance (often radioactively labelled) is allowed to penetrate the skin at a predetermined area for a certain time period. Afterwards the skin is gently washed to remove remaining unabsorbed test substance on top of the skin. The method then involves sequentially removing of microscopic layers of the exposed stratum corneum by repeated application and removal of adhesive tape. The shedded cells attached to the tape are then analysed using an appropriate analytical method.
The method is inexpensive, uncomplicated and a minimally invasive method given that only dead cells (corneocytes) embedded in their lipid matrix are removed.
In dermatopharmacology tape stripping is used to assess cutaneous drugs in the skin after topical dermatological treatment. Tape stripping is a particularly helpful technique to assess the local bioavailability of drugs whose target site is the stratum corneum itself, like e.g. antiseptics (Lboutounne et al., 2002) , antifungal drugs (Alberti et al., 2001b;Alberti et al., 2001a;Pershing et al., 1994) or UVA/UVB filters (Fernandez et al., 2002;Jacobi et al., 2004;Sarveiya et al., 2004;Wissing & Muller, 2002).
The tape stripping method has some disadvantages (see below) but is useful when it comes to studying diseased vs. healthy skin (Jakasa et al., 2004), chemicals that accumulate in the skin, or when it comes to comparing in vitro and in vivo data (Reddy et al., 2002).
4.2.4 Advantages/disadvantages
There are always ethical considerations when choosing an in vivo experimental method. The main advantage is that an in vivo technique uses a physiologically and metabolically active system (OECD, 2004).
The disadvantages when using microdialysis are the cost of pumps, probes and involvement of participants/volunteers. When choosing microdialysis the volunteers have a limited participation time before it becomes uncomfortable to continue staying in the same position. Other considerations are how the test substance interacts with the probe - if it sticks to the probe membrane or not, if the membrane’s permeability - “cut-off” - is suitable for penetration of the specific substance and whether the test substance is suitable for microdialysis or not according to the lipophilicity of the substance – since very lipophilic substances prefer to stay outside the probe. Moreover, a wide range of toxicants will for ethical reasons not be eligible for in vivo human experiments.
Tape stripping is a simple, inexpensive and non-invasive method that can be used in humans as well as in animals. The variation in models used is considerable as the number of tape strips used in removing the stratum corneum varies with the type of tape (Bashir et al., 2001), pressure applied during application and force in removal. Also biological factors give a variation such as anatomical location of application, sex, ethnicity and age of the subject (Palenske & Morhenn, 1999;Loffler et al., 2004). Tape stripping as a stand-alone technique is therefore mainly helpful to assess the local bioavailability of drugs whose target site is the stratum corneum itself.
4.3 In vitro models versus in vivo models
By using an in vitro model the ethical topics are minimized since the use of human volunteers or animals are excluded and the donor skin samples would have been sent to destruction anyway.
The method is excellent looking at the permeability properties of the skin, since they can be maintained when the skin is removed from the body by excision and therefore the stratum corneum, with its principal barrier function, is kept intact.
Other advantages of the in vitro model are the possibilities to replicate the experiment with samples from the same person. Also different species can be studied under identical laboratory conditions and enable comparisons within and between species.
So far it has not been possible to identify any active transport through the skin and since the transportation is believed to be passive diffusion, the barrier that the stratum corneum (non-viable epidermis) consists of is maintained and reliable for in vitro penetration studies.
As mentioned above, the in vitro models are inexpensive to maintain and have limited time consumption.
Results from the in vitro model are easier to reproduce and the method has fewer restricted parameters causing variations. Good association with in vivo experiments has been shown and the method is suitable to predict human transdermal absorption (Scott et al., 1992;van Ravenzwaay B. & Leibold, 2004)}. When choosing an in vitro system with continuous sampling as is seen in the flow-through model, lag-times are often easier to measure, and will therefore not have to be estimated based on a back extrapolation from the linear part of the penetration curve, as is necessary when using the static diffusion cells. Several in vitro models are accepted by the OECD guidelines and qualitative agreement between models is good. Quantitative differences will exist due to e.g. differences in skin thickness between full-thickness skin and dermatomed skin.
In vivo models, however, are the gold standard since they operate using living tissue and operate in a physiologically and metabolically active system. Often the only real limitation is the ethical considerations when using this model.
5 Biological factors influencing skin absorption
Many factors are known to influence dermal absorption. First of all the site of application/exposure is very important when it comes to skin penetration, just as the age of the person exposed has an effect on the amount of substance penetrating the skin.
As quite a few studies have been made using skin from different animals, the knowledge that there is a significant difference in absorption when it comes to animals and humans has led to the necessity of a thorough interpretation, if adapting data from animal studies to be used in relation to humans.
Also the state of the skin is important when using it for experimental research. It is fundamental to evaluate the barrier function as the integrity of this parameter, e.g. the hydration of the skin, is very essential to the experiment. Since it is known that the skin has its own metabolism even though it is very low compared to metabolism in the liver, this must also be considered when making skin penetration studies where the absorption of a specific substance is being explored.
5.1 Anatomical site
The site of exposure has proved to be of significance in the penetration of many substances and there is not a complete pattern of regional absorption variation that accounts for all substances. Yet there is a general pattern shown by Feldman and Maibach (1967) in a penetration study of hydrocortisone. Here the skin on the scrotum had the highest permeability and the increasing rate over the areas was as follows: plantar < palmar < back < scalp < axilla < forehead < scrotum. The penetration rate from the foot to the scrotum varied 42-fold (Feldmann & Maibach, 1967). Another study demonstrated a lower permeability across abdominal skin than leg skin. The different order of absorption in this study demonstrates that the variation between areas is unaffected by the thickness of the skin in the particular site (Elias, 1981). The explanation is not quite clear. Other influencing factors are the number of follicles, the thickness of the stratum corneum, the sebum composition as well as the distance of capillaries to the surface of the skin (Rougier et al., 1999).
5.2 Age
Age has an influence on the skin and also on the penetration through the skin. The skin structure changes with increasing age. The stratum corneum becomes drier as the activity of the sebaceous glands decreases and the surface lipids diminish. Some have pointed towards a marked age-related decrease in skin lipids, at least up to age 50 years (Rogers et al., 1996), although others indicated sparse or no relationship (Cua et al., 1995;Schreiner et al., 2000). The amount of collagen decreases and becomes less soluble in chronologically/intrinsically aged skin, but becomes thickened and more soluble in photoaged areas. Intrinsic aging also slowly degrades elastin which accumulates as damaged elastin. Increased synthesis of abnormally structured elastin occurs in photoexposed areas. In general, age leads to increased folding and decreased interaction of proteins with water. Thus, although aged skin holds an increased amount of water, the majority of this is tied to itself in tetrahedral form, rather than being bound to proteins (Waller & Maibach, 2006). Also the blood supply is reduced as the capillary network degenerates. This has shown to be most effective on hydrophilic substances whereas very lipid-soluble substances are able to dissolve into the stratum corneum even when the accessible surface lipids are reduced (Roskos et al., 1989).
5.3 Barrier function
The barrier functions depend mainly on the integrity of the stratum corneum. Changing or damaging the skin structure increases the permeability. The permeability can be affected chemically (detergents, solvents), physically (weather, occlusion, sunlight) or pathologically (mechanical damage, disease). A number of detergents, alcohols and solvents have been shown to alter the barrier integrity by changing the properties of the barrier (Nielsen & Nielsen, 2000;Dias et al., 2008;Rosado & Rodrigues, 2003;Kezic et al., 2001). Several studies have been made where the skin was damaged in different grades. Thus, Bronaugh and Stewart (1985) used abraded, UV-radiated and tape-stripped skin to demonstrate an increasing absorption from < 2 to > 100-fold, depending on the degree of damage being done to the skin (Bronaugh & Stewart, 1985b). Tape-stripping is a mechanical method that is used to remove the stratum corneum. After tape-stripping the permeability coefficient of morphine is seen to increase several hundred fold compared to intact mouse skin. Absorption of fentanyl and sufentanil is increased more than 40 times (Roy et al., 1994). In an in vivo study using volunteers and microdialysis, the absorption of salicylic acid was highly enhanced (150 times) in a tape-stripped skin (Benfeldt et al., 1999).
Also diseased skin can cause an inherent skin barrier defect and studies have shown that patients suffering from skin diseases like atopic dermatitis or lamellar ichthyosis have reduced or altered lipid contents in their stratum corneum (Imokawa et al., 1991;Yamamoto et al., 1991). The changed lipid composition causes abnormal lipid organization in the stratum corneum (Pilgram et al., 2001). Lately Jakasa et al. have shown an altered penetration profile of SLS and polyethylene glycol into the stratum corneum (SC) of patients with atopic dermatitis (AD) compared to control subjects. This indicates that even non-involved skin in patients with AD has altered barrier characteristics, emphasizing the importance of skin protection and prevention of skin contact with chemicals (Jakasa et al., 2006a;Jakasa et al., 2007). Another recent study reports that about 10% of people of European ethnicity are carriers of loss-of-function mutations in the filaggrin gene (Palmer et al., 2006). Filaggrin is a key protein of the SC that assists terminal differentiation of the epidermis and creation of the skin barrier. Different types of mutations in the filaggrin gene lead to damaged barrier formation, which manifests as altering degrees of dry skin (Kezic et al., 2008), ichthyosis (Chen et al., 2008), and/or dermatitis (Nomura et al., 2007; de Jongh et al., 2008). Additionally, as a precursor of amino acids and derivatives that act as a “natural moisturizing substance” filaggrin is largely responsible for the ability of SC of the skin to stay hydrated at low environmental humidity (Rawlings & Harding, 2004; Kezic et al., 2008). The above results do not give quite enough quantitative information and more specific research is needed. This will be described later.
5.4 Species
There are significant differences in the dermal absorption in animals and in humans. Differences in the lipid content, structure and thickness of the stratum corneum are significant factors (Walters & Roberts, 1993). Further, laboratory animal skin has more appendages than human skin which can be the reason for increased transdermal absorption. A range of experimental studies in vitro as well as in vivo have been published. Most of them have an acceptable internal validity, but clearly need an interpretation before being used for human risk assessment. A recent study has also questioned the reliability of converting percutaneous absorption data from rats to humans due to the mentioned differences in species as they studied the absorption of hazardous substances (Korinth et al., 2007a).
5.5 Metabolism
The primary metabolic organ of the human body is the liver. The skin, however, also maintains a certain metabolic capacity. It contains enzymes which can be very active in degradation of penetrating substances (Denyer S.P et al., 1985). Enzymes can catalyse both endogenous agents such as steroids and hormones, and xenobiotics such as pharmaceuticals and environmental chemicals. Most substances that are absorbed across the skin barrier have a reasonable lipophilicity. The role of the enzymes is to detoxify and to increase polarity and thereby produce more water-soluble products that are more easily eliminated from the body. But the enzymes can also activate the molecules to more toxic metabolites as was shown by Liu et al. where carbosulfan and furathiocarb were metabolized to the more toxic carbofuran (Liu et al., 2002;Liu & Kim, 2003). The balance between cutaneous activation and detoxification is a critical determinant of systemic exposure in humans (Hotchkiss SAM, 1998).
The activity of skin metabolism is very low compared to the hepatic activity. Skin metabolism may, however, be important if large surface areas are exposed. The degree of metabolism largely depends on the enzymes involved. Esterase is very active in the skin whereas cytochrome P450 enzymes are not. Therefore metabolism of chemicals, which are primarily metabolized by P450 enzymes will hardly be affected by skin metabolism (Sartorelli et al., 1997).
5.6 Hydration
To give the skin a good barrier function the hydration of the skin needs to be balanced, and a certain quantity of water is needed. If the hydration increases the permeability may be enhanced manyfold. Increased skin hydration is often seen in occlusive environments, such as in the use of protective gloves or working in a humid environment like dishwashers, hairdressers, cleaners etc. These occupations are associated with high prevalence of contact dermatitis which has been associated with enhanced penetration of skin irritants through hydrated skin. Since occlusion has proved to be the single factor which increases skin penetration the most it is of great significance to avoid chemicals inside a glove or other equipment (Wester & Maibach, 1983). In a study of medical drugs the occlusion of the application area resulted in hydration of the tissue. Consequently, the skin got swollen and wrinkled. The temperature increased at the same time and thereby increased the permeability with up to 300-fold (Varvel et al., 1989). See section 10.4.3.
6 Pesticides
6.1 History
Even earlier than 2500 BC, humans have utilized pesticides to protect their crops. The first known pesticide was sulphur dusting used in Sumeria about 4500 years ago. By the 15th century, toxic chemicals such as arsenic, mercury and lead were being applied to crops to kill pests. In the 17th century, nicotine sulphate was extracted from tobacco leaves for use as an insecticide. The 19th century saw the introduction of two more natural pesticides, pyrethrum which is derived from chrysanthemums, and rotenone which is derived from the roots of tropical vegetables (Miller, 2002).
In 1939, Swiss chemist Paul Müller revealed DDT as a very efficient insecticide. DDT rapidly became the most used pesticide in the world. However, in the 1960s it was discovered that DDT was preventing many fish-eating birds from reproducing, which posed a significant risk to biodiversity. DDT was also found to cause birth defects in animals and humans. DDT is now banned in most industrialized countries, but is still used in some developing nations to prevent malaria and other tropical diseases by killing mosquitoes and other disease-carrying insects.
German scientists experimenting with nerve gas during World War II produced the organophosphorous insecticide parathion, marketed in 1943 and still commonly used today. Throughout the 1950s and 60s, these types of chemicals became major pest control agents.
“Silent Spring”, Rachel Carson's landmark challenge to the abuse of synthetic pesticides, was published in 1962 and initiated the movement towards agrochemical regulation that is still fiercely debated (Nationmaster, 2003).
Pesticide use in Denmark has decreased during the last decade. Data from the Danish Environmental Protection Agency show that the pesticide sale has decreased from 19,400 ton in 1995 (6,600 ton active ingredient) (Miljøstyrelsen, 1998) to 12,234 ton in 2006 (3,200 ton active ingredient) (Miljøstyrelsen, 2007).
Pesticides of today are designed to persist for shorter periods of time in the environment and are supposedly less lethal than the early days of calcium arsenate and DDT. There might even be evidence for the fact that alternatives to pesticides can be more effective then the use of chemicals. Sweden has reduced its use of pesticides by half with hardly any reduction in crops. In Indonesia, farmers have reduced pesticide use on rice fields by 65% and experienced a 15% crop increase (Miller, 2004).
Today the most frequently used pesticides are non-persistent organophosphates, including glyphosate (the active ingredient in Roundup), which is currently the world's most used herbicide (Nationmaster, 2003).
6.2 Definition
A pesticide is a substance or mixture of substances used for preventing, controlling, or lessening the damage caused by a pest. By their very nature, most pesticides create some risk of harm. Pesticides can cause harm to humans, animals, or the environment as they are designed to kill or otherwise adversely affect living organisms. At the same time, pesticides are useful to society. Pesticides can kill potential disease-causing organisms and control insects, weeds, and other pests (US Environmental Protection Agency, 2007).
The use of pesticides is a way to control organisms which are considered harmful such as mosquitoes that can spread potentially lethal diseases like malaria and insects that can cause allergic reactions. Insecticides can protect animals from illnesses caused by parasites like fleas. Pesticides can prevent sickness in humans caused by diseased products. Pesticides are used in grocery stores and food storage facilities to manage rodents and insects that infest food such as grain, but each use of a pesticide carries some associated risks. Correct pesticide use decreases these risks and is therefore of great importance.
6.3 Use
Pesticides such as rodenticides, herbicides, fungicides and insecticides are used not only to prevent harmful organisms in occupational areas, but also in private homes where pesticides are used in the garden fighting weeds, ants, bees etc., and some like malathion are used directly on humans against lice.
Rodenticides are chemicals intended to kill rodents. Rodents include mice, rats, squirrels, chipmunks etc. The poison is used in households as well as in agriculture. It is most effective if tasteless and odorless in lethal concentrations and if it has a delayed effect. Rodenticides are e.g. Kiltin Bromanol B 100 (bromadiolon) or Frunax-D (difenacoum).
Herbicides are used to kill unwanted vegetation. They can destroy specific targets while leaving the desired crop relatively unharmed. Some herbicides work by restricting the growth of the weed by interfering with the plant hormones. Other herbicides are non-selective and kill all plant material with which they come into contact. Herbicides are e.g. Roundup (glyphosat), or NF-M 750 (MCPA).
Fungicides are chemical compounds used to avoid the spread of fungi in gardens and crops, which can cause harm to the plants. Fungicides are e.g. Octave (paclobutrazol) or Tachigaren 70 WP (hymexazol)
Insecticides are used against insects.They target the insect organism in all developmental forms from eggs and larvae to insects. Insecticides are used in agriculture, medicine and the household. Practically all insecticides have the potential to change ecosystems; several are toxic to humans and mammals and can therefore alter the food chain. Agricultural needs must be considered together with environmental and health issues when using insecticides. Insecticides are e.g. Pirimor (pirimicarb), Cygon (dimethoate) or DDT (dichlordiphenyltrichlorethan).
6.4 Toxicity
A toxin is often referred to as a toxic substance which is naturally produced where as a toxicant is a toxic “human-made” substance. The distinction is not always clear.
Toxic substances are classified in many ways, depending on the classifiers – their needs and interests. Substances can be discussed in terms of their use, target organ, source and effects.
Pesticides achieve desired effects but also have a spectrum of undesired effects. These effects are refered to as the adverse, deleterious, or toxic effects of the pesticides.
Toxicity can be divided into immediate or delayed toxicity and reversible or irreversible toxic effects.
Immediate toxic effects can be defined as those that occur or develop rapidly after a single administration of a substance, whereas delayed toxic effects are those that occur after some time.
Whether an effect is reversible or irreversible often depends on the ability of the injured tissue to regenerate. Some tissues are more susceptible to injury and the regeneration is therefore different. The liver has a high ability to regenerate as opposed to the CNS. Injuries to the two different organs therefore have very different outcomes and the damage is referred to as reversible and irreversible (Klaassen CD, 1996).
The undesired effects of pesticides may also be divided into different targets:
- Developmental effects - ability to affects fertility or foetal development.
- Carcinogenicity - ability to produce cancer or to assist carcinogenic chemicals.
- Mutagenicity - ability to cause genetic changes.
- Liver damage - death of liver cells, jaundice (yellowing of the skin), fibrosis and cirrhosis.
- Reproductive disorders - such as reduced sperm count, sterility, and miscarriage.
- Neurotoxicity - including accumulative effects on cholinesterase depression associated with organophosphate insecticides.
- Allergenic sensitization - development of allergies to pesticides or chemicals used in formulation of pesticides.
These undesired effects can either be produced immediately or delayed and be reversible or irreversible. As an example teratogenicity and carcinogenicity caused by pesticides are usually considered irreversible toxic effects.
6.5 Symptoms
Symptoms based on toxicity may be shown:
- As acute toxicity. Thus, exposure to a pesticide may cause acute effects such as nausea, chest pain and vomiting as well as chronic effects resulting from kidney, liver and lung damage.
- As a slowly progressive form of toxicity without any previous clinical signs of acute intoxication. An example could be breathing difficulty or skin sensitization (allergy) following repeated exposure to a pesticide.
- As the occurrence of a disease or condition initiated by previous exposure. Delayed appearance of neurotoxicity and development of cancer years after a period of exposure are organophosphates (neurotoxicity).
7 Effect of solubility and molecular size on skin penetration
- 7.1 Determining percutaneous penetration rate
- 7.2 Relation between Kρ and logPow
- 7.3 Association between lag-time and solubility
- 7.4 The relation between the lag-time and the molecular weight
- 7.5 Skin deposition and substance solubility
- 7.6 Molecular size in relation to substance solubility and deposition
- 7.7 Exposure in relation to occupational risk assessment
The dermal absorption is influenced by the solubility of the pesticide - a high lipophilicity as well as a high hydrophilicity limit the skin penetration (McDougal & Boeniger, 2002). This is an important consideration when designing drugs for topical use, cosmetics and also when designing pesticides. Pesticides need an effective penetration when it comes to the outer membrane of the target organism (plants or pests). Knowledge about the fastest or optimal penetration is also significant when estimating human risk and the ability to secure prevention.
Substances that are intended to cross the skin must have a molecular weight below 1000 daltons. The size is probably not the limiting factor but with increasing size the chemical structure becomes more complex, the partitioning behaviour changes and penetration is therefore reduced. The skin is a multi-complex membrane and changes from an avascular and lipophilic structure (stratum corneum) to a more aqueous structure (the viable epidermis and dermis). Uncomplicated penetration of a substance requires both solubility in the lipophilic environment and the more aqueous environment (Guy et al., 1987).
7.1 Determining percutaneous penetration rate
The objective of this part of the report is, through results on percutaneous penetration of different test substances, to determine an interval in penetration ability in relation to the substance solubility and hopefully find an interval where the most effective percutaneous penetration takes place. The selected substances represent a broad interval of solubilities as well as a relevant interval in molecule weights.
Nielsen et al. have tested glyphosate, benzoic acid, malathion, caffeine, pirimicarb, methiocarb, paclobutrazol, dimethoat and prochloraz and characterized these substances by their solubility, logPow, molecular weight, Kρ and lag-time as shown in Table 1 (Nielsen JB et al., 2006;Nielsen et al., 2004). The potential to create a reservoir is in the table indicated by the amount of substance in the epidermis and dermis at the end of experiments. The total penetration is together with the unabsorbed amount of test substance in the donor chamber, and the amount remaining in the skin.
Experimental data on percutaneuos penetration rate (flux) are obtained by measurements of concentrations in the receptor chamber over time. This measurement is used to generate the apparent Kρ-(permeability coefficient) by dividing the steady-state flux obtained in experiments with infinite dosing with the concentration in the donor chamber. For occupational risk assessment following dermal exposure, the flux or relative absorption (percentage absorption per day of an occupationally relevant dose) is often used to estimate the risk. The penetration rate is, however, not in its own sufficient to evaluate the toxicity profile of a pesticide after dermal exposure. Thus, two pesticides with significantly different maximal flux, and therefore also different Kρ’s, may cause identical pesticide doses given their lag-times also differ. Except for in vitro studies with continuous sampling (flow-through cells), lag-times are often difficult to measure and will most often be estimated based on a back extrapolation from the linear part of the penetration curve (see Figure 4 in section 3.2.1).
Table 1. Solubility and penetration characteristics of 9 test substances. Experiments were based on static diffusion cells mounted with human skin, carried out under comparable conditions, and terminated at 48 hours.
| Relative deposition | ||||||||||
| Solubility | logPow | MW | Kρ | lag-time | receptor | epidermis | dermis | donor | recovery | |
| (g/L) | (g) | (um/h) | (h) | (%) | (%) | (%) | (%) | (%) | ||
| Glyphosat | 12 | -1.7 | 170 | 0.06 | 7.9 | 0.4 | 0.5 | 0.2 | 91.0 | 92.0 |
| Caffein | 21.7 | 0.16 | 194 | 3.8 | 6.9 | 18.7 | 3.4 | 3.6 | 72.8 | 99.6 |
| Dimethoate | 23.8 | 0.7 | 229 | 4.9 | 22.3 | 5.1 | 0.4 | 1.5 | 104.5 | 109.4 |
| Pirimicarb | 3 | 1.7 | 238 | 27.7 | 15.2 | 34.1 | 1.5 | 9.7 | 52.5 | 96.3 |
| Benzoic acid | 3 | 1.83 | 122 | 50.6 | 1.5 | 93.4 | 0.4 | 1.1 | 4.2 | 99.0 |
| Malathion | 0.15 | 2.75 | 330 | 1.9 | 2.9 | 11.6 | 2.7 | 5.7 | 71.4 | 91.4 |
| Paclobutrazol | 0.026 | 3.2 | 294 | 29.2 | 18.4 | 23.5 | 3.7 | 17.0 | 41.1 | 84.1 |
| Methiocarb | 0.027 | 3.34 | 225 | 38.6 | 11.7 | 50.9 | 2.4 | 23.5 | 17.1 | 94.4 |
| Prochloraz | 0.034 | 4.4 | 377 | 17.8 | 21.1 | 13.7 | 4.2 | 27.5 | 58.3 | 101.7 |
The diffusion process across the skin is believed to be passive and the experimental conditions should reflect infinite dosing. Thus, the maximal flux characterizes a steady-state situation and will therefore represent the slowest of the two rate constants for penetration (in or out of the skin compartment). These rate constants depend on molecular size and lipophilicity of the substance as well as the lipophilicity difference between the matrix that the substance is leaving to the matrix that the substance is entering. If the test substance has a preference for the skin compared to the receptor, a higher concentration gradient will be needed to get substantial transfer into the receptor compartment, and the potential for a reservoir exists. The lag-time is therefore also depending on the existence of a reservoir in the skin barrier, whereas the flux in infinite dose studies at steady state will be independent of the existence of a reservoir effect. Moreover, the thickness of the skin membrane affects the distance that a substance will have to pass through and thus the lag-time.
7.2 Relation between Kρ and logPow
The most efficient absorption is a combination of high penetration rate, illustrated as a high Kρ, and a short lag-time. The amount of substance found in the skin - the epidermis and dermis, is likewise of importance as this reflects the amount of substance which is available for subsequent penetration.
The results from the studies by Nielsen et al. (2004, 2006) show that the lowest penetration coefficient (Kρ) reflects the most hydrophilic substance (glyphosat) and the hydrophilicity of the substance decreases as the Kρ gets higher (Table 1). The maximum Kρ is seen for benzoic acid with a Kρ almost a 1000-fold higher than Kρ of glyphosate. The difference in hydrophilicity expressed as the octanol/water ratio (Pow) is more than 3000 which corresponds to a difference in logPow of just around 3. As for the 3 most lipophilic test substances (methiocarb, paclobutrazol, prochloraz), the Kρ is lower than for benzoic acid. The decrease of logPow in relation to benzoic acid is, however, not as significant for the most lipophilic substances as for the most hydrophilic substances. There is a clear relation between logKρ and logPow. (Figure 11).
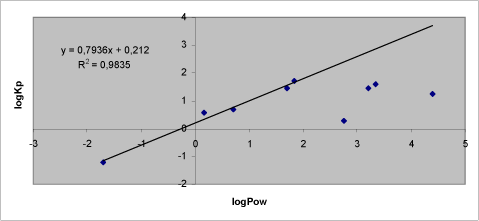
Figure 11. Correlation between logPow and logKρ for nine test substances. Trend line is based on chemicals with logPow below 2.
This is in good agreement with previous studies describing the optimal penetration through the skin of substances with a logPow around 2 (Morgan et al., 2003;Cross et al., 2003) and a recent study demonstrating a fast penetration into the lipophilic stratum corneum but then a more resistant penetration into the underlying hydrophilic part of the skin by didecyldimethylammonium chloride logPow 4.7 (Buist et al., 2007).
In their previous studies Nielsen et al. did not demonstrate a clear relation between molecular weight and penetration rate expressed as Kρ. This is rather surprising given previous literature studies based on larger databases of studies involving dermal penetration, which show a direct relation between molecular weight and penetration rate (Magnusson et al., 2004;Tsai et al., 2003). However, when taking a closer look at these studies of 87 test substances they show large variations and quite a few of the substances are removed from the regression analysis because of deviation compared to the other substances in the studies. In comparison to the studies of Nielsen et al. in 2004 and 2006 who found one substance (malathion) with an unexpected penetration profile, they may well have found other deviating substances had they included more substances in their study. If they had done so they might have found an association between their results and the Australian literature study by Magnusson et al. (Magnusson et al., 2004).
Based on the solubility and molecular weight of malathion, it was expected that the penetration characteristics would place this substance between methiocarb and benzoic acid. As seen from Figure 11 and Table 1, this is not the case. A thorough examination of the experimental set-up, analytical method etc. has revealed no obvious explanation. The most likely explanation is that structural circumstances cause the deviating penetration characteristics of malathion compared to other substances.
7.3 Association between lag-time and solubility
The amount of a substance that in a given time is absorbed through the skin is also affected by the lag-time of the substance. The lag-time will depend not only on the solubility but also on the size of the substance. There is a relationship between lag-time and solubility of a substance. A substance with a fast penetration rate is also expected to pass through the skin after a shorter lag-time than a substance with a slow penetration rate (Figure 12 and 13) (Nielsen JB et al., 2006).
Figure 13 indicates that a short lag-time is to be expected of substances with water solubilities between 3g/L and around 20g/L.
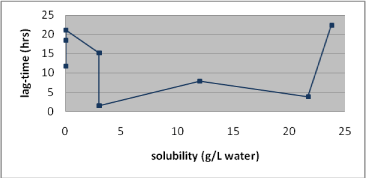
Figure 12: Association between the solubility in water and the lag-time of nine test substances.
7.4 The relation between the lag-time and the molecular weight
There is a clear relationship between the molecular weight and observed lag-time of most of the test substances in the study by Nielsen et al. When the molecular weight increases the lag-time does the same (Figure 13) (Nielsen JB et al., 2006).
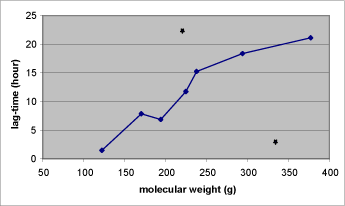
Figure 13: Relation between the molecular weight and the lag-time of the nine test substances described in Table 1. The lag-time of malathion (below) and dimethoat (above) is marked with stars.
The smallest test substance in the study by Nielsen et al. had a molecular weight of 122 g and showed a lag-time of 1½ hours. In comparison with substances with weights above 350 g a clear increase in lag-time was observed. The lag-time increases to more than 20 hours for some of the substances with high molecular weights. The significant difference in lag-time affects the amount of a substance penetrating in a limited period of time. This is highly relevant in occupational risk assessments since skin absorption on a normal working day of 6-8 hours will depend on the substance lag-time.
When evaluating the molecular weight in relation to the lag-time it is also here found that malathion acts unexpectedly by having a lag-time of only 3 hours when having a weight of 330g – a lag-time which would be expected of a substance with half the weight (Nielsen JB et al., 2006).
7.5 Skin deposition and substance solubility
The deposition in the epidermis is not surprisingly relatively limited since it is only the stratum corneum that is involved. The differences in epidermis deposition indicate a relatively high deposition of the most lipophilic substances even though caffeine differs by a relatively high deposit of around 3%.
In the dermis there is a greater relative deposit of the lipophilic substances which was also to be expected as the dermis is a predominant lipophilic matrix. Analogous results are shown in a study including 5 different alcohols with different solubility characteristics (Cross et al., 2003).
The amount of test substance deposit in the donor chamber after end exposure time is as expected greatest among the more hydrophilic substances as they show poor affection of the more lipophilic compartments in the skin (Figure 14).
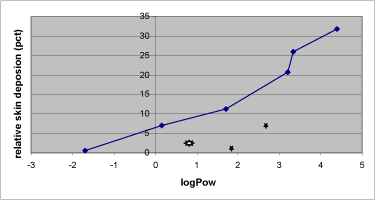
Figure 14: Association between the lipophilicity expressed as logPow and the relative skin deposition of the nine test substances in Table 1. Excluded from the line drawing is from left to right: dimethoate, benzoic acid, malathion.
Even though dimethoat has the same molecular weight as methiocarb, the solubility characteristics of dimethoat were a 1000-fold higher water solubility. Not only does the high water solubility prevent a dermal penetration, but it also prevents a significant amount of pesticide to even enter the more lipophilic epidermis. Despite of the high water solubility in pimicarb, this substance had difficulty depositing in the skin compartment, even though it did penetrate the skin with great flux when compared with methiocarb having the same weight as pirimicarb. Thus, 13% of the pirimicarb dose penetrated the dermal barrier in the experiment with 24 h pesticide exposure when compared with only 8.7% of the methiocarb dose. The high flux and low skin deposition of pirimicarb, when compared with methiocarb, signify the lack of a longer lasting skin reservoir of pirimicarb, and a limited accumulation during the penetration process. This conclusion is based on the observation that penetration into the receptor chamber continues for at least 6 h after pirimicarb is removed from the donor chamber, the low flux during the last part of the experimental period, and the very low skin deposition at 48 h (Nielsen et al., 2004). These observations on skin deposition are also in agreement with the lower lipophilicity of pirimicarb when compared with methiocarb and the relatively low lag-time compared with the other pesticides.
7.6 Molecular size in relation to substance solubility and deposition
The influence of molecular size has been compared using three pesticides (methiocarb, paclobutrazol and prochloraz) with similar solubilities, but different molecular weights (Nielsen & Nielsen, 2000;Nielsen et al., 2004). The flux and consequently the calculated Kρ values for methiocarb and paclobutrazol were almost identical irrespective of whether the exposure period was 6 h or 24 h, whereas flux as well as Kρ for prochloraz (the pesticide with the highest molecular weight) was lower.
The amount of pesticide penetration during the 48 h experimental period decreased with increasing molecular weight irrespective of exposure periods. Likewise, a significantly (P< 0.0 l) higher fraction of the administered dose was removed from the donor chamber in experiments with methiocarb (Mw = 225 g/mol) when compared with paclobutrazol (Mw = 293 g/mol). The significantly higher removal of pesticide from the donor chamber was also reflected through a higher skin deposition in experiments with methiocarb when compared with experiments with paclobutrazol. Prochloraz, on the other hand, had the lowest total penetration but a distribution between donor and skin compartments that was more like methiocarb than paclobutrazol, although prochloraz had the largest molecular weight. Despite equal solubilities in water, the logPow value of prochloraz was higher than logPow for methiocarb and paclobutrazol, which could indicate a higher reservoir preference for prochloraz when compared with the more hydrophilic donor and receptor solutions. The apparent reservoir preference of prochloraz is most significantly reflected through the distribution in the experiment with 24 h exposure, where more than 90% of the administered dose was estimated to remain in the skin compartment when the experiment terminated after 48 h (Nielsen et al., 2004).
Based on the data from Nielsen et al. 2004, it can be concluded that solubility characteristics significantly affect penetration rates as well as skin deposition, and that a too high as well as a too low lipophilicity may limit the rate and degree of skin penetration. These observations pertain to studies on full-thickness skin, in which a significantly larger potential for skin deposition (reservoir) exists. Several mathematical models have been developed to describe the relationship between dermal penetration and the underlying physical parameters such as logPow and molecular weight (Guy & Potts, 1993;McKone & Howd, 1992;Wilschut et al., 1995). Equal for these theoretical models is their unidirectional dependence on molecu1ar weight and logPow as well as their lack of dependence upon experimental conditions related to skin thickness and choice of receptor fluids. The observation that a too high as well as a too low lipophilicity limits dermal penetration is thus at variance with those models. A possib1e explanation might be that these models do not sufficiently consider the penetration as a process involving penetration from a hydrophilic donor to a more lipophilic membrane as well as from the lipophilic membrane and into a more hydrophilic receptor. In the first process, penetration of hydrophilic compounds (like dimethoat) will be limited, and in the second process, more lipophilic compounds (like prochloraz) will prefer to remain in the lipophilic skin compartment.
7.7 Exposure in relation to occupational risk assessment
To mimic an occupational exposure situation Nielsen et al. have studied skin penetration after 6 h exposure time (Nielsen et al., 2004). The use of infinite dosing is at variance with most occupational exposures, but is not important for the study of temporary deposition in the skin and subsequent penetration to the receptor. As the lag-times for most pesticides tested were more than 6 hours, it was not surprising that the maximal fluxes for these pesticides obtained in the 6-hours exposure experiments were generally lower than in the 24-hours exposure experiments, whereas pirimicarb with a short lag-time had comparable Kρ values in experiments with 6 hours as well as 24 hours. An important observation was that most of the applied dose of the two most hydrophilic pesticides (pirimicarb and dimethoat) was recovered in the donor wash after 6 h, which implies that preventive measures in the form of handwash will significantly reduce the skin penetration of these pesticides following occupational exposures. However, an equally important observation was that dermal penetration continues long after exposure has ended due to absorption of a temporarily deposited pesticide present in the skin compartment at the end of exposure.
Nielsen and Nielsen have also studied the penetration of methiocarb, paclobutrazol and pirimicarb and lag-times between 10 and 18 h were reported (Nielsen & Nielsen, 2000) A later study by the same group found lag-times significantly deviating from these earlier observations. The receptor fluid did, however, differ between the two studies. Thus, a more physiological receptor (5% BSA in a 0.9% NaCl aqueous solution) was used in the later study when compared with the use of 50% ethanol in the earlier study. These differences make direct comparisons difficult as ethanol is known to significantly affect the barrier characteristics of the skin membrane (Nielsen, 2000). In general, these observations are in agreement with other studies demonstrating the influence of the vehicle on dermal penetration characteristics (Singer & Tjeerdema, 1993;Baynes et al., 2002;Brand & Mueller, 2002).
As illustrated in Fig. 2, different combinations of flux and lag-time may cause identical amounts of a chemical to cross the dermal barrier at a certain point in time. Thus, the total amount of a chemical penetrating during a certain time period is not very informative on its own. This is especially relevant in relation to short-term exposures such as most occupational exposures, where exposure time will often be less than or close to the experimental lag-time. Under these circumstances, an exposure assessment based on measurement of blood concentration at the end of a work shift will ignore pesticide deposited in the skin and demonstrate zero absorption and thus underestimate the actual exposure. Although literature indicates that the systemic concentration of a number of pesticides tested did not increase after exposure was ended (Zendzian, 2003), the continued presence of pesticide in the blood compartment indicates continued absorption from the dermal reservoir and thus a continued rise in systemic dose. As a prolonged presence of a lower concentration of a toxicant may be equally important as a short exposure causing a high blood concentration, we suggest that data used for regulatory agencies should include steady-state flux (or Kρ) and lag-time as well as an estimation of the potential importance of the skin reservoir.
8 Effects of detergents on skin integrity and penetration
8.1 Surfactants
Surfactants are a large group of chemical substances also known as surface-active agents or detergents. They are characterized by their ability to reduce the surface tension on hydrophilic solutions. They will allow lipophilic substances to mix with hydrophilic solutions and let hydrophilic substances penetrate lipophilic membranes. Surfactants are broadly classified as anionic, cationic, amphoteric or non-ionic, according to the nature of the hydrophilicity yielded in aqueous solution (Effendy & Maibach, 1995). The anionic surfactants are normally considered most aggressive on the skin barrier, but also nonionic surfactants have proved to cause a great increase in penetration (Nielsen et al., 2000).
The use of specific detergents in pesticides is conditional of technical characteristics in relation to the use of the products. Detergents with identical or comparable technical qualities sometimes have completely different toxicity characteristics (Scheuplein & Bronaugh, 1983). So far there has been no sign of a direct relation between the quantitative ability to reduce surface tension and the potential to damage the skin membrane (Klaassen CD, 1996).
Pesticides are not used commercially as pure chemicals, but are mixed with different detergents to change solubility characteristics and in some cases increase the penetration into plant leaves. Some detergents are known to affect the barrier function of the skin. In a study by Buist et al. it was demonstrated that a single as well as a repeated exposure to specific biocidal products significantly increased skin permeability, especially when the detergents were used undiluted (Buist et al., 2005). The commercial formulation of furathiocarb had a higher skin penetration rate than the technical furathiocarb (Liu & Kim, 2003), probably because the commercial formulation contains detergents which affect the skin permeation.
When assessing an occupational risk it is therefore essential to be aware of the influence that the detergents have on the skin barrier function and not only evaluate the effect of the active ingredient.
8.2 Effect of detergents on skin integrity
In 2004 Nielsen made a comparison study for the Pesticide Office in the Danish Environmental Protection Agency. This study was based on the most used detergents or co-surfactants in Denmark stated by the Pesticide Office; propylenglycol, ethylenglycol and lignosulphonic acid (Nielsen JB, 2004).
The results of the study are listed in Table 2. The control substances in the study were SLS as positive control substance and water as negative control substance. SLS is a well known skin barrier disrupter (Benfeldt & Serup, 1999;Benfeldt et al., 1999;Okuda et al., 2002) and has shown an increase in water evaporation, a decrease in the thermal transition of the lipids and a disturbed diffraction pattern by SAXS (small angle x-ray scatter) (Ribaud et al., 1994). SLS is a known component in soap and therefore a relevant substance to explore. Water is known not to cause any barrier disruption.
Also Nonyl-Phenol-Ethoxylat (NPE) was tested since NPE and similar polyethoxylates have been widely used as detergents in pesticide formulations (Dooms-Goossens et al., 1989) and are known to change the barrier properties of human skin in vitro (Dooms-Goossens et al., 1989;Nielsen et al., 2000). Thus, NPE has recently been demonstrated to facilitate and enhance the dermal in vitro penetration of tritiated water by 60% (Nielsen, 2000). Data from other experiments show different percutaneous penetration characteristics when NPE is added to the donor phase. NPE reduced the dermal penetration by 40-50% for paclobutrazol and pirimicarb, whereas the percutaneous penetration of methiocarb was reduced less significantly. An increase in the concentration of NPE showed no change in the effect observed on the penetration (Nielsen & Andersen, 2001). Table 2 show that NPE used in low doses does not affect the capacitance even though the penetration of tritiated water is doubled (the capacitance indicates if the skin is able to separate electrical charge. Skin samples with a high capacitance are unable to act as a capacitor, which means that the skin barrier is damaged). NPE increases the capacitance but only when applied in high concentrations. In high concentrations the capacitance as well as the penetration is doubled.
Ethanol is an often used detergent known to increase the penetration of several substances (Krishnaiah et al., 2008;Obata et al., 1993;Takahashi et al., 1991). A recent study of hydrocortisone penetration through canine skin showed a significantly higher maximum flux when hydrocortisone was dissolved in ethanol (Mills et al., 2005). As seen in Table 2 the penetration of tritiated water increases 180% but without affecting the capacitance. Both ethylenglycol and propyleneglycol showed no effect on the capacitance but the penetration was doubled and tripled respectively (Nielsen JB, 2004). A similar result was seen in a study by Mills et al. where the penetration of testosterone through canine and equine skin was tested and a significantly higher flux was found for the drug dissolved in a vehicle containing ethanol or propyleneglycol (Mills et al., 2006;Mills, 2007). Lignosulphonic acid showed no effect on the capacitance and no increase in the penetration of water even at high concentrations. None of the detergents (ethylene-glycol, propylene-glycol and lignosulphonic acid) damaged the skin integrity significantly following 48 hours of exposure. The glycols did, however, increase the penetration of water.
Table 2. The results from studying the effect of detergents on skin integrity. The capacitans have been measured at the start of the experiment, after 24 hours and again at the end of the experiment. The penetration of tritiated water was estimated after 48 hours (Nielsen JB, 2004).
| Detergent | Capacitans (nF) | Penetration of H2O (% of water) |
|||
| Name | conc. (mM) | t = 0 hours | t = 21 hours | t = 48 hours | |
| Water (negative control) | 40 | 43 | 65 | 100 | |
| SLS (positive control) | 7 (0.2%) | 46 | 345 | 840 | 340 |
| 35 (1.0%) | 27 | 461 | 7180 | 410 | |
| Ethanol (24%) | 34 | 40 | 46 | 180 | |
| Nonyl-phenol-ethoxylat | 2.5 | 37 | 44 | 58 | 190 |
| 10 | 47 | 109 | 124 | 220 | |
| Ethylen-glycol | 2.5 | 44 | 53 | 57 | 220 |
| 10 | 42 | 44 | 52 | 110 | |
| Propylen-glycol | 2.5 | 55 | 61 | 74 | 290 |
| 10 | 43 | 46 | 50 | 110 | |
| Lignosulphonic acid | 0.25 | 33 | 34 | 35 | 66 |
| 1.0 | 41 | 44 | 47 | 89 | |
9 Concomitant exposure to pesticides and detergents
- 9.1 Approval of commercial products
- 9.2 Penetration profile of commercial products vs. the active ingredient
- 9.3 Mixture of pesticides
- 9.4 Conclusion
9.1 Approval of commercial products
Hazard assessment of pesticides needs to evaluate the toxicity of the active substances as well as the detergent. In this context it is essential also to assess the potential of the detergents to act as mediators of absorption of other potentially toxic substances. Mediators can change the absorption by an increase in the bioavailability of certain products (Sartorelli et al., 1997) or by a direct effect on the skin barrier function (Treffel P & Gabrad B, 1996;TupkerRA, 1990).
Many experiments deal with percutaneous penetration of pesticides, but focus on the active ingredient and do not test the commercial products which are often a mixture of an active substance and a detergent. It is, however, the commercial products that are of interest, since the combination of ingredients may change the penetration characteristics (Baynes & Riviere, 1998;Baynes et al., 2002). Approvals of various compounds and recommendations for protective equipments are often based alone on data from these studies, which as will be shown later can be misleading. Also the US EPA Interagency Testing Committee (ITC) mandated by the Toxic Substances Control Act has required industry to measure in vitro penetration of several chemicals in their pure or neat form (EPA, 2004).
9.2 Penetration profile of commercial products vs. the active ingredient
A comparison study of different commercial products and their active ingredients has been carried out. The penetration rate, lag-time and amount of penetrated substance have been studied and compared by Nielsen (Nielsen JB, 2004).
The commercial product Mesurol® (used to kill snails and slugs in ornamental plants and vegetables) and the active ingredient methiocarb have been studied and the graphs in Figure 15 picturing penetration over time demonstrate significant differences between the product and the active ingredient (Nielsen JB, 2004).
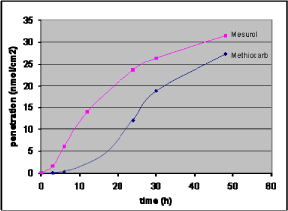
Figure 15: Penetration of Mesurol and the active substance methiocarb. Mesurol as well as methiocarb is applied to the donor chamber in a methiocarb concentration of 0.2 mM. The points represent a mean of 9-11 single data values.
The graph also shows also that the lag-time is reduced from 10 hours to only two hours in the penetration of the commercial product. The decrease in lag-time causes a considerable increased penetration over time and therefore a risk of absorbing larger amounts of chemicals during normal working conditions than assumed based on studies of the active ingredient alone. The knowledge of the difference in lag-time is valuable in relation to advice and guidance related to hygiene and the use of protective gloves when using this pesticide.
The commercial product PirimorG® (used to kill greenfly in agriculture, greenhouses and nurseries) and the active ingredient pirimicarb have been studied and the lag-time of PirimorG® was found to be 2 hours (Table 3), which is significantly lower than the 9 hours observed for the active ingredient (Figure 16).
Table 3: Dermal penetration of PirimorG (pirimicarb) as well as the effect of Mesurol on dermal penetration of PirimorG. The results of penetration and flux are given as mean +/-SEM.
| Pesticid e | N | Penetration of PirimorG after 48 h (nmol/cm²) |
Flux of PirimorG (nmol/cm²/h) |
Lag-time (hrs) |
| PirimorG | 9 | 23.86 ± 2.80* | 0.69 ± 0.11 | 2.1 |
| PirimorG + Mesurol | 12 | 30.64 ± 1.47* | 0.95 ± 0.08 | 4.7 |
* - significant differences; Students t-test, p<0.01.
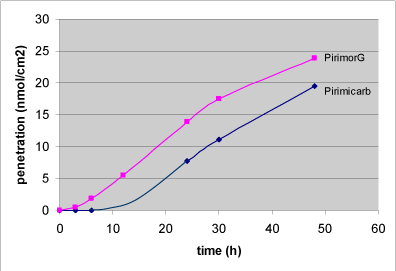
Figure 16: Penetration of PirimorG and the active substance pirimicarb. PirimorG as well as pirimicarb were applied in the donor chamber in a pirimicarb concentration of 0.2 mM. The points represent a mean of 9-11 single data values.
The penetration rate, however, is similar in both substances, thus only 25% more active substance penetrates the skin in 48 hrs when testing the commercial product compared to the pure active substance. But as mentioned before the reduced lag-time is highly relevant when looking at risk assessment and working conditions.
The condition of the substance may also be important when evaluating permeation of chemicals. Most laboratory studies measure chemical penetration from an aqueous solution through isolated human or animal skin, although most exposures are not from pure aqueous solutions. Frasch et al. have measured skin permeability and lag time for three neat chemicals of industrial importance (diethyl phthalate (DEP, slightly volatile), 1,2-dichloroethane (DCE, highly volatile), and naphthalene (NAP, solid)) and found the maximum measured flux of saturated aqueous DEP was ~ 2 times greater than that from neat DEP but lower with DCE. Maximum flux of neat DCE was nearly 6 times higher than DCE in a saturated aqueous buffer. The maximum penetration rate of solid NAP powder was ~ 2 times higher than the rate from a saturated aqueous solution (Frasch et al., 2007). NAP is known to be a strong skin irritant (IPCS INCHEM, 2002), which may be the reason for the increased permeation rate. Studies on other chemicals have, however, not been able to demonstrate particular penetration differences between saturated aqueous solutions and powder (McCarley & Bunge, 2003;Romonchuk & Bunge, 2006).
9.3 Mixture of pesticides
Commercial formulations contain detergents and other compounds to increase absorption by targeted plants or to ease mixing and distribution in aqueous solutions. These chemicals, however, are also potential penetration enhancers for human skin. A mixture of PirimorG® and Mesurol® give us an indication of how two substances may influence each other. The results are shown in Tables 4 and 5 and the interaction between the two products is absolutely diverse.
Table 4: Dermal penetration of Mesurol measured as methiocarb, as well as the effect of PirimorG on dermal penetration of Mesurol. Results on penetration and flux are given as mean +/-SEM.
| Pesticide | N | Penetration of Mesurol after 48 h (nmol/cm²) |
Flux of Mesurol (nmol/cm²/h) |
Lag-time (hrs) |
| Mesurol | 9 | 31.44 ± 2.46 | 1.39 ± 0.22 | 1.9 |
| Mesurol + PirimorG | 12 | 32.51 ± 1.16 | 1.25 ± 0.15 | 3.4 |
Table 5: Effect of combined exposure of methiocarb (0.2mM) or pirimicarb (0.2mM), as well as the effect of three detergents in relation to the dermal penetration and flux of paclobutrazol (0.2mM). Results are given as mean +/-SEM.
| Pesticide exposure |
Detergent | N | Penetration of methiocarb after 48 hrs (nmol/cm²) |
Flux of methiocarb (nmol/cm²/hr) |
Lag-time (hrs) |
| Paclobutrazol (paclo) | - | 17 | 12.09 ± 1.57* | 0.37 ± 0.04* | 15.3 |
| Paclo+ methiocarb | - | 11 | 9.28 ± 0.96 | 0.38 ± 0.06 | 17.0 |
| Paclo+ pirimicarb | - | 11 | 21.51 ± 1.47* | 0.73 ± 0.09* | 13.1 |
| Paclo+ methiocarb | PG | 10 | 11.08 ± 1.23 | 0.35 ± 0.03 | 10.1 |
| Paclo+ methiocarb | SLS | 9 | 10.12 ± 0.94 | 0.34 ± 0.05 | 12.9 |
| Paclo+ methiocarb | EG | 9 | 9.39 ± 0.83 | 0.28 ± 0.05 | 11.8 |
| Paclo+ pirimicarb | PG | 9 | 18.13 ± 1.65 | 0.65 ± 0.08 | 13.5 |
| Paclo+ pirimicarb | SLS | 10 | 18.55 ± 1.77 | 0.56 ± 0.07 | 10.9 |
| Paclo+ pirimicarb | EG | 10 | 19.78 ± 2.73 | 0.58 ± 0.10 | 11.7 |
PG – Propylene glycol (10mM); SLS – Sodium Lauryl Sulphate(7mM); EG – Ethylene glycol (10mM). * - significant differences; Students t-test, p<0.001.
PirimorG® did not affect the penetration of methiocarb from Mesurol® whereas the opposite is the case when it comes to penetration of PirimorG®. There is a clear change in penetration characteristics showing a 40% increased flux of pirimicarb in combination with Mesurol®. This gives a 30% increased penetration after 48 hours. The reason for this increase in flux should probably be found in the effect of the detergents present in the commercial products since it cannot be detected in the studies of the pure active substances. There is a good correlation between these findings and the changes that was found in the studies of the pure active substance compared to the commercial products where a clear increase in penetration was also found (Nielsen JB, 2004).
The data demonstrate that some substances can increase the permeability of others, as well as decrease the lag-time. The increase in penetration of active substances when testing commercial products is in good correlation with other studies of chlorpyrifos, atrazine, arachlor and trifluralin, where a significant increase in dermal penetration of the four pesticides are seen when they are administered as commercial products (Brand & Mueller, 2002;Griffin et al., 2000)
The evidently shorter lag-time seen in the studies of Mesurol® and PirimorG® signifies that under normal working conditions the absorption of those two substances happens faster than originally assumed given that earlier studies are made on active substances alone and not the commercial products.
But also other kinds of compounds applied to the skin before handling pesticides can change the permeation as seen in a recent study made by Brand et al.(Brand et al., 2007). They tested four commercially available moisturizing creams and their capacity as transdermal penetration enhancers using the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) as a model compound. Their data demonstrated that pre-treatment with three of the four creams increased the absorption of 2,4-D as evidenced by either an increased cumulative penetration or shorter lag-times. Also skin barrier creams have demonstrated to enhance the penetration rates of different industrial solvents in a study by Korinth 2003 and the creams significantly enhanced the penetration rates of solvents from complex mixtures compared with the single solvents. (Korinth et al., 2003a).
9.4 Conclusion
It is known that the nature of the vehicle in which a substance is applied to the skin may change the absorption rate, lag-time or both by either increasing it or slowing it down. The reduced lag-time demonstrated the range of effects that different detergents may have on penetration of pesticides, and suggests that regulatory agencies consider these data when evaluating pesticides resubmitted for approval after a change in formulation. In most cases the Danish EPA does not require updated pesticide-specific penetration data when re-evaluating known pesticides in changed formulations, but rely on extrapolation from other products as well as physic-chemical comparisons between detergents. This procedure is complicated and requires considerable toxicokinetic as well as chemical insight. The EPA should consider how large differences can be before submission of supporting data on penetration should be required.
To the people handling these products, e.g. greenhouse workers, farmers, house and garden owners, this information is also important knowledge and advice and guidance must be carefully considered so that these people take the necessary precautions, e.g. hygiene and the use of gloves as protective equipment.
10 Penetration of pesticides through slightly damaged skin
- 10.1 OECD guidelines
- 10.2 Work-related skin problems
- 10.3 Trans Epidermal water Loss (TEWL)
- 10.4 Skin-damaging factors
- 10.5 Affecting skin integrity
- 10.6 Conclusion
The more we know about the ideal and optimal situation, the more important our knowledge about the exceptions get. Because many people have a decreased integrity of their skin barrier, it is important to adjust the experimental studies to real-life situations and use experimental models where it is possible to study percutaneous penetration through slightly damaged skin (Kezic & Nielsen JB, 2008). A minor damage to the skin is expected to increase the penetration, but whether this is caused by a decreased lag-time or an increased penetration rate (flux) needs further investigation. It is also relevant to study whether an effect on penetration might be observed to the same extent in more or less hydrophilic or lipophilic pesticides, though recent data on a number of test substances indicate the effect of lipophilicity on permeability characteristics (Borras-Blasco et al., 2004). In other words, the presence of a mixture of detergents/vehicles, as in the formulated pesticide products (sales formulations), may influence penetration characteristics as indicated through accessible data on the effect of detergents on the integrity of the skin barrier (Dupuis et al., 1986;Nielsen et al., 2000;Nielsen, 2000).
10.1 OECD guidelines
The most reliable information on dermal absorption for the use of human health risk assessment is achieved from occupational field studies and controlled exposure studies in volunteers. However, due to technical and ethical restrictions these studies are limited.
Therefore, in experimental percutaneous penetration studies it has over the years been common to use a number of different techniques and methodologies based on skin samples from humans (cadavers or surgical waste) or animals (rats or pigs). Recently guidelines have been drawn towards more standardised methods, and the latest guideline from Organisation for Economic Cooperation and Development (OECD) is one of the outcomes (OECD, 2004). The main purpose of guidelines are to assure an acceptable quality in the output of an experiment and by that the possibility to compare results from different laboratories. A general characteristic of guidelines on experimental studies of percutaneous penetration, including the latest from OECD, is the attempt to aensure intact, homogenous, and optimal barrier conditions of the skin samples used. Thus, specific requirements are listed to ensure the integrity of the skin barrier, which must be ensured and documented through measurement of trans-epidermal water loss (TEWL), electrical resistance or capacitance (OECD, 2000;Davies et al., 2004). Thus, the OECD guidelines used in hazard and risk evaluations prescribe experimental conditions with optimal barrier integrity of the skin, which in many occupational settings probably is not true.
10.2 Work-related skin problems
The skin exposure in daily work – household or occupational situations - is not always ideal. Skin problems are among the most dominant reasons for absence from work in many countries. Occupational contact dermatitis is the most frequent occupational skin disease with an estimated average incidence rate of 0.7-1.5 cases per 1,000 workers per year according to a German study (Elsner, 2007). In another German study the prevalence of damaged skin was close to 70% in the printing industry and approximately 80% among workers in the rubber industry (Korinth et al., 2005;Korinth et al., 2007b;Korinth et al., 2007c). Yet another study from Finland finds an incidence of occupational diseases of 5.9 cases per 1000 person-years among machinists and 2.7 cases per 1000 person-years in the total workforce. Of these occupational diseases in machinists 27% were skin-related problems (Suuronen et al., 2007).
Epidemiological studies indicate that only 4-9% of people with work-related skin problems are on the sick list and the true prevalence of skin disorders within the active work force is believed to be a lot higher than official statistics (Diepgen, 2003;Hadgraft, 2004). A Danish study describes an incidence of work-related dermatological diseases in 17,700 people out of a total work force of 2.6 mio. (Halkier-Sorensen, 1996), thus, 6.8 cases per 1000 persons. The number of self-reported cases of hand eczema in a hospital population showed an overall frequency of hand eczema in 12 months of 22.8% in an unselected population of hospital employees in a recent Danish study (Flyvholm et al., 2007).
Data from the U.S. point out that one fourth of nurses met the criteria for currently damaged skin on the hands (Larson et al., 1997), and the damage has proven to be related to use of gloves and handwashing practices.
A Swedish study showed that occupational dermatological problems often end up as chronic conditions with a majority reporting symptoms at a 12-year follow-up. A skin disease influences the occupational situation for the majority (82%) and for 15% results in exclusion from the labour market through unemployment or disability pension (Meding et al., 2005).
These few examples illustrate the magnitude of the problem in high risk occupations with skin exposure to solvents, water and detergents.
Affected skin barrier due to continuing wet work, small bruises, skin irritation, eczema etc. has proven to increase dermal absorption (Bronaugh & Stewart, 1985b;Grandjean P, 1990;Ilyin et al., 1975;Scott & Dugard, 1986). Accordingly, industrial hygienists have for several years anticipated, and advised workers, that a decreased integrity of the skin barrier may increase percutaneous penetration of chemicals (Grandjean P, 1990;Levang et al., 1999).
10.3 Trans Epidermal water Loss (TEWL)
Damage of the skin barrier implies an increase in TEWL. TEWL is the normal, constitutive loss of water vapour from the skin in absence of sweat gland activity (Gioia & Celleno, 2002). In a number of studies good correlation between TEWL and drug penetration of different substances has been demonstrated (Benfeldt et al., 1999;Tsai et al., 2001). In a recent study on the validity of TEWL, comparisons to a variety of methods in a variety of models demonstrated that TEWL was a reliable tool for the assessment of variations in permeability and barrier function (Fluhr et al., 2006).
However, Chilcott et al. reported that TEWL did not reflect permeability of sulphur mustard - a lipophilic substance (Chilcott et al., 2002) and Tsai et al. showed absence of correlation between TEWL and dermal absorption of highly lipophilic substances (estradiol and progesterone) in acetone pre-treated skin (Tsai 2001). Benfeldt et al. found a 2.2-fold increase in dermal absorption of salicylic acid through acetone-treated skin although TEWL was not different from untreated skin (Benfeldt et al., 1999). Regardless of a poor correlation between TEWL and dermal absorption for some lipophilic substances described in different studies it is suggested that TEWL may be used as a predictor of barrier integrity and dermal absorption of hydrophilic and slightly lipophilic substances (Levin & Maibach, 2005).
TEWL has shown to be useful in studies evaluating different skin types/thicknesses or barrier qualities. A recent study compared heat-separated epidermis and dermatomed skin in a qualitative and quantitative percutaneous absorption study and used TEWL as endogenous standard (Atrux-Tallau et al., 2007).
10.4 Skin-damaging factors
10.4.1 Detergents
Detergents are also known as surfactants and are described in section 8.1. They can enhance skin permeability by a range of mechanisms, together with enhanced solubility and increasing partitioning into the stratum corneum. Certain anionic surfactants such as sodium lauryl sulphate (SLS) affect not only the barrier itself but during prolonged exposures also the underlying viable cell layers leading to skin inflammation and possibly further deterioration of the skin barrier. The effect of SLS on the skin penetration has been shown to depend on the lipophilicity of the penetrant (see below in section 10.4.1)
10.4.2 Organic solvents
An organic solvent is a (carbon-containing) chemical that dissolves a solid, liquid, or gaseous solute, resulting in a solution. They are widely used as degreasers, cleaning agents, solvents for plastics, paints and rubber in numerous industrial applications. Data from England estimate that two million workers have regular contact with solvents (Semple 2004). Solvents are responsible for as much as 20% of all cases of occupational dermatitis because of their severe effect on the skin (Kamijima et al., 2007). The effects are local irritant reactions, epidermal necrosis and cytotoxicity associated with oxidative stress (Rowse & Emmett, 2004).
Often exposures to solvents occur when skin contacts a liquid, but also exposures to vaporous mixtures have shown to damage the skin barrier (Huss-Marp 2006).
10.4.3 Hydration of the skin
To keep a good skin barrier and an optimized barrier function it is important that the skin is sufficiently hydrated. When the skin becomes dry the flexibility and elasticity of the skin decreases. This can lead to cracks in the stratum corneum and a vulnerable skin barrier. In some occupations, such as in lithium battery manufacturing and the pharmaceutical industry a low room humidity under 40-50% is required. Working in this environment for a long time may cause dry and xerotic skin (Sato et al., 2003).
Also increased hydration may alter the skin permeability (Warner et al., 2003). This may occur following working in a wet environment, which is the case for health care personnel, housekeepers, hairdressers and dishwashers who are in frequent contact with water. But also conditions where normal water evaporation is prevented by wearing of gloves and protective clothing will increase skin humidity. These occupations are known to have a high prevalence of contact dermatitis.
Depending on the air humidity, the water content of stratum corneum is around 40% of the tissue dry weight (Williams & Barry, 2004). When occluded the stratum corneum will absorb a lot more and this can affect the permeability of the skin to chemicals (Rawlings & Harding, 2004;Rawson et al., 2005;Scheuplein & Blank, 1971;Warner et al., 2003;Wester & Maibach, 1983). Idson (1971) claimed that increasing skin hydration enhances the absorption of all substances that go through the skin (Idson, 1971). However, increased hydration (due to occlusion) does not always increase penetration rates. Bucks et al. (1991) stated that hydration reduced the penetration rate of hydrophilic compounds like hydrocortisone (Bucks et al., 1991). In contrast, Wurster & Kramer (1961) observed that occlusive covering that prevented water loss at the same time increased the dermal absorption of some hydrophilic compounds. Increased skin hydration has been mentioned as the likely cause of the increase in absorption, although in most studies contributions from potentially misleading things such as increased temperature or accumulation of sweat cannot be rejected (Fluhr et al., 1999;Wurster & Kramer, 1961;Zhai & Maibach, 2001).
Jones et al. showed that dermal absorption of 2-butoxyethanol in humans was enhanced significantly when air humidity increased from 40% to 65% (Jones et al., 2003). Dermal absorption of propoxur at different levels of humidity between 50 and 90% showed a linear relationship between the level of air humidity and the level of skin humidity (Meuling et al., 1997). Also different parabens (methyl-paraben and butyl-paraben) have an increased absorption during occlusion (Akomeah et al., 2004).
10.4.4 Mechanical damage
Damage to the skin can also be done mechanically by abrasion, scrubbing and skin friction and will result in disruption of the skin barrier. To imitate mechanical skin damage the stratum corneum may be removed by tape-stripping (see chapter on Methods to study dermal penetration). Morgan et al. found in a microdialysis study a correlation between the extent of damage to the stratum corneum and the penetration of hydrophilic aciclovir and penciclovir. A total removal of the stratum corneum increased the penetration by 400-1300-fold in contrast to normal skin (Morgan et al., 2003). In another microdialysis study Benfeldt et al investigated the penetration of the hydrophilic salicylic acid and showed an increased absorption of 150-fold in stripped skin (Benfeldt et al., 1999). Akomeah et al. studied the effect of abrasion induced by a rotating brush on the skin permeation of solutes with varying physiochemical properties and found a significant increase in permeability on especially the hydrophilic substances (Akomeah et al., 2008).
Furthermore, penetration of lipophilic substances is also affected by a damaged skin barrier. Less than 1% penetration was observed with intact skin, whereas up to 23% of latex proteins applied to abraded skin was recovered in the receptor fluid within 24 h of exposure (Hayes et al., 2000).
10.4.5 Pathologically compromised skin barrier
Compromised skin barrier can be an inherent skin barrier defect. Suffering from atopic dermatitis and lamellar ichthyosis will change the skin profile with altered fatty acids and ceramide content (Imokawa et al., 1991;Imokawa, 2001;Yamamoto et al., 1991). Also a mutation in filaggrin which is a key protein of the stratum corneum may alter the skin barrier (Palmer et al., 2006;Chen et al., 2008). (see section 5.3).
The majority of children suffering from atopic dermatitis demonstrate filaggrin mutations (Palmer et al., 2006). An increase in the occurrence of this phenomenon over the last decade of 15-20% is a huge concern (Vasilopoulos et al., 2004). As a result of alterations in barrier function, it is possible that larger substances such as allergens and irritants may enter the skin (Proksch et al., 2003).
Patients suffering from atopic dermatitis have an altered skin barrier not only in the inflammatory area but also in the skin with a clinically normal appearance (Seidenari & Giusti, 1995). Furthermore, de Jongh revealed a higher permeability in individuals with atopic constitution but without manifestation of atopic dermatitis (de Jongh et al., 2006).
Also Jakasa et al. showed a higher diffusion of SLS and polyethylene glycol of a different molecular size through not affected skin in patients suffering from atopic dermatitis and demonstrated a relative increase in penetration after damage to the skin barrier in the glycol with the highest molecular size (Jakasa et al., 2006b;Jakasa et al., 2007). A study of offset printing workers exposed to a glycol ether also demonstrated that erythema and scaliness were important parameters affecting skin absorption (Korinth et al., 2003b). Patients suffering from impaired genetic inclination to produce increased levels of stratum corneum or suffering from an imperfection in enzymes causing premature breakdown of corneodesmosome will experience damage of the stratum corneum (Cork et al., 2006).
10.4.6 Distribution within the skin
Test substances are expected to move between compartments based on plain diffusion along a concentration gradient, though movement from one compartment to another will also depend on the lipophilic character of the two compartments. If the skin compartment is more lipophilic than the solution in which the substance is dissolved, lipophilic substances are expected to prefer to stay in the skin compartment, whereas more hydrophilic substances may prefer to stay in the solution. If the concentration gradient becomes sufficiently large, the hydrophilic substance might get forced into the skin membrane, but then instantly continue to the more hydrophilic receptor chamber. In contrast, lipophilic substances will be expected to stay in the skin compartment until the concentration gradient is large enough for the substance to pass through. In a medical situation we might want to create a substance that will penetrate the skin into the general circulation. To do this the substance needs to be lipophilic enough to want to pass into the skin membrane and also be hydrophilic enough to want to leave the skin and enter into the bloodstream.
These aspects may partly explain the prolonged lag-time of hydrophilic substances as well as the absence of any significant penetration of lipophilic substances. It is therefore important in experimental studies not to underestimate the consequence of the lipophilicity in the receptor phase.
Lag-time depends on the distance that a substance needs to move to get from the donor chamber to the receptor chamber. The distance is due to the lamellar structure of the stratum corneum considerably longer than the straight measurable thickness of the stratum corneum (Borras-Blasco et al., 2004). By changing the lamellar structure or the physiochemical characteristics of the stratum corneum it is therefore possible to manipulate the lag-time and the flux. Flux at steady state will, however, as previously mentioned also depend on the rate constant for transport from donor into the skin compartment and from the skin out into the receptor phase.
Changing the structure may reduce the distance across the skin membrane but may not necessarily affect the rate of transfer. Dermal penetration modifiers may in other words affect lag-time and flux separately as well as together (Figure 17).
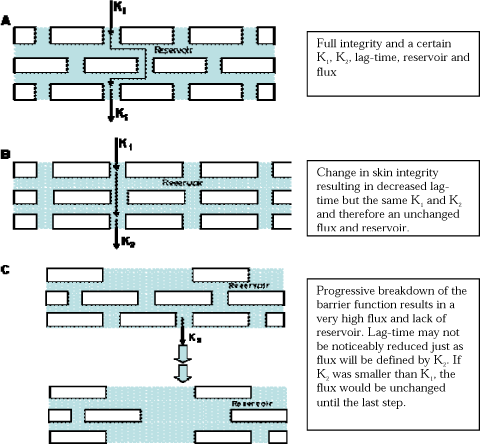
Figure 17. Changes in skin barrier leads to changes in permeability as visualised in Figures A, B and C.
Commercial products usually contain a number of detergents that act as modifiers of solubility and/or penetration characteristics of the active ingredient. Experiments have shown that detergents presented in commercial products can act by enhancing the maximal flux without having any affect on the lag-time or decrease the lag-time without affecting the flux (Nielsen, 2005a).
Sodium lauryl sulphate (SLS) is another modifier that acts through changes in the skin integrity. The effect of pre-treatment with SLS is generally an increased maximal flux and a reduced lag-time. The increased flux is, however, only significant for the most hydrophilic test substances (Borras-Blasco et al., 2004;Nielsen, 2005a).
10.5 Affecting skin integrity
Inducing a universal impairment of the skin integrity should on one hand encourage a measurable, stable and permanent damage, but on the other hand not compromise the integrity to a degree that will take away all barrier properties.
Compromised integrity of the skin may be achieved chemically as well as mechanically through continual tape-stripping or abrasions by use of a scalpel blade or sand paper (Ilyin et al., 1975;Scott et al., 1986;Proksch et al., 1996).
A well-known treatment of the skin in order to produce a damage of the barrier is to treat the skin for a specific time period with sodium lauryl sulphate (SLS). This method is more reproducible than consecutive tape-stripping. SLS is often used as a positive control in studies on irritation or effects of chemical exposure on the barrier integrity of the skin (Dickel et al., 2004;Nielsen, 2000) as well as used as a penetration enhancer in drug formulations (Piret et al., 2000;Nokhodchi et al., 2003;Borras-Blasco et al., 2004). Damage of the skin barrier integrity by SLS has previously been attributed to removal of intercellular hydrophilic lipids, which may be observed through an increase in TEWL. (Froebe CL 1990). A later study has indicated that SLS fluidizes the lipid bilayers in the stratum corneum (Ribaud et al., 1994). These two effects are suggested to increase percutaneous penetration of primarily hydrophilic compounds (Akomeah et al., 2007;Akomeah et al., 2008), whereas the percutaneous penetration of highly lipophilic compounds should remain unaffected (Borras-Blasco et al., 2004). It has been demonstrated that concentrations below 1% SLS are sufficient to produce an impaired barrier function (Figure 19) and that an increase in SLS concentration above 1% will not further enhance percutaneous penetration of lipophilic test substances (Borras-Blasco et al., 2004;Nielsen, 2000). By using SLS it is easy to establish reproducible skin damage and the use of different SLS concentrations has the advantage of inducing different degrees of damage.
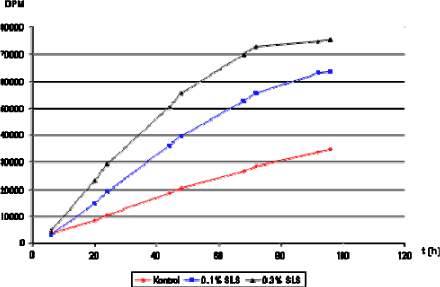
Figure 18. Penetration of tritiated water after pre-treatment with SLS for three hours in different concentrations (0.1% and 0.3%). The concentration of tritiated water was identical in all three groups and is expressed as disintegrations per minute (DPM).
Studies have shown an intraexperimental coefficient of variation between 11% and 16% which is an acceptable reproducibility, especially when the natural heterogeneity among human skin samples is considered (Nielsen, 2005).
Lag-time and flux determine the amount of chemical that may be observed penetrating the skin within a certain time period. Both parameters have previously been demonstrated to depend on the lipophilicity of the test substances used in studies on percutaneous penetration of skin with full integrity (Nielsen et al., 2004). Nielsen et al. 2006 demonstrated an increase in maximal penetration rate leading to an increased penetration coefficient (Kρ) in 6 test substances. The increase in Kρ of benzoic acid is not significant, which is probably because benzoic acid already penetrates undamaged skin easily, has a low molecular size and therefore has no obvious gain of the skin barrier being compromised.
More lipophilic substances – like malathion and methiocarb - show an increase in Kρ of 90% and 15% respectively (Nielsen JB et al., 2006)
The difference in penetration rate through undamaged versus damaged skin may be due to the fact that malathion has a larger molecule size and therefore acquires greater advantage when the skin barrier is damaged than a chemical with a small molecule size will (illustrated in Figure 19 and Table 6 (Nielsen et al., 2007).
Table 6: Penetration characteristics of six test substances from Nielsen et al. 2006. The results are stated as mean values of data from 11-15 penetration cells.
| Intact Non-washed |
Damaged Non-washed |
Intact Washed |
Damaged Washed |
Sales product Intact Non-washed |
Sales product Damaged Non-washed |
|
| Kρ (um/h) | ||||||
| Glyphosate | 0,04 | 0,97 | 0,04 | 0,07 | ||
| Caffeine | 4,5 | 21,7 | 2,5 | 14,3 | ||
| Dimethoate | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzoic acid | 51,1 | 53,8 | 39,5 | 60,7 | ||
| Malathion | 4,5 | 8,4 | 4,6 | 11,6 | ||
| Methiocarb | 39,5 | 45,5 | 30,0 | 35,0 | 47,0 | 35,5 |
| Lag-time (h) | ||||||
| Glyphosate | 3,3 | 8,7 | 4,2 | 4,1 | ||
| Caffeine | 6,5 | 5,9 | 5,0 | 4,8 | ||
| Dimethoate | - | - | - | - | - | - |
| Benzoic acid | 1,8 | 1,7 | 1,7 | 1,5 | ||
| Malathion | 2,6 | 2,2 | 3,0 | 2,6 | ||
| Methiocarb | 15,7 | 11,7 | 14,3 | 8,5 | 13,8 | 10,9 |
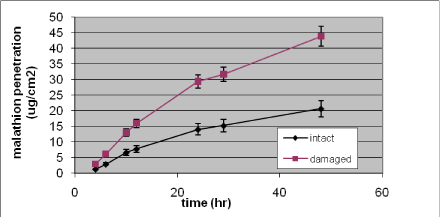
Figure 19. In vitro percutaneous penetration of malathion through intact and slightly damaged human skin. A total amount of 424 µg (2 mg/ml) was added to the donor chamber and penetration followed for 48 h. Results are presented as mean ± SEM (n = 13–14 per group)
A clearer increase in penetration rate is seen in the hydrophilic substances. An example is the graphs below where a 500% increase in penetration rate of caffeine is seen and an increase of 25-fold in penetration rate of glyphosate is also demonstrated (Figures 20 and 21) (Nielsen et al., 2007). Jakasa et al. came to the same conclusion when studying the permeability of PEG in compromised skin (Jakasa et al., 2006b).
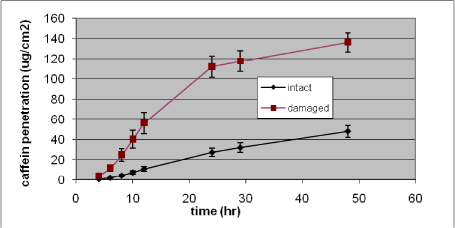
Figure 20. In vitro percutaneous penetration of caffeine through intact and slightly damaged human skin. A total amount of 424 µg (4 mg/ml) was added to the donor chamber and penetration followed for 48 h. Results are presented as mean ± SEM (n = 14 per group)
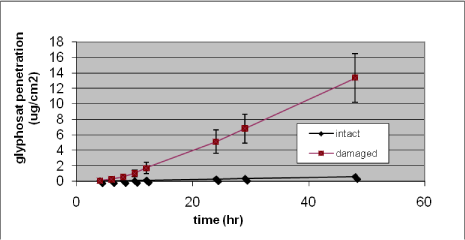
Figure 21. In vitro percutaneous penetration of glyphosate through intact and slightly damaged human skin. A total amount of 424 µg (4 mg/ml) was added to the donor chamber and penetration followed for 48 h. Results are presented as mean ± SEM (n = 13–14 per group)
10.6 Conclusion
Damage of the skin undoubtedly has an effect on the permeability coefficient (Kρ), penetration rate, lag-time as well as the total penetration of chemicals with different solubilities. An increase in permeability coefficient Kρ is most significant for those substances that due to their physicochemical characteristics (the most hydrophilic as well as the most lipophilic) have low penetration rates through intact skin. Substances having a hard time penetrating intact skin will in slightly damaged skin achieve a greater gain in their penetration characteristics. The hydrophilic compounds will be those most affected by a change in skin barrier integrity since it is the step from the donor chamber into the more lipophilic skin that is affected. This part of the absorption is uncomplicated to lipophilic substances.
This significant increase in penetration of especially the hydrophilic substances may well have the consequence that exposed employees, suffering from damaged skin, will absorb considerably more through the skin than traditional in vitro experiments have predicted.
The knowledge of impaired skin integrity of many exposed employees and also of people in households should clearly be considered when setting standards for dermal exposure to chemicals. Furthermore, SLS is extensively used as a detergent in many products including household products and soaps. Even in small concentrations the effect of SLS is obvious and this knowledge should motivate producers of these products to use other detergents that to a much lesser degree affect the skin barrier.
11 Skin wash and temporary skin deposition
The reservoir effect has previously been described (Chapter 3.2.5) and is defined as a substance staying and accumulating in the skin instead of passing directly through to the bloodstream. The substance may then be released to the bloodstream with a certain delay. The absorption of the substance into the bloodstream will continue from the application site. This happens at a gradually declining pace, giving the appearance of prolonged elimination (Cnubben et al., 2002). Another possibility is that the substances remaining in the upper layers of the skin are removed by washing the skin or by desquamation after end of exposure.
When it comes to pesticide exposure and risk assessment it is important to know if it is possible to terminate the absorption of some of the pesticide residue by simple handwash; e.g. if the amount of pesticide remaining in the stratum corneum should actually be thought of as absorbed or whether it can be washed out. A guideline describing experimental methods in skin penetration expresses the fact that the amount of pesticide remaining in the skin at the end of an experiment should be considered absorbed (OECD, 2000). There may, however, be differences in hydrophilic and lipophilic substances just as there may be differences when it comes to damaged skin or intact skin as previously described (Chapter 10).
11.1 Skin deposition
After end of dermal exposure a large part of the pesticide is deposited in the skin. Part of the substance is placed in the upper layers (stratum corneum/epidermis) and some in the lower dermis. Regulations have up till now been based on the assumption that all pesticide residing in these layers is considered absorbed. A study from 2003 carried out on rats tested 19 different pesticides and found an ongoing absorption after skin wash in 17 of 19 pesticides (Zendzian, 2003), but only half of the pesticides gave rise to an increased systemic concentration (Zendzian, 2003). Transfer of these observations in rats to the human exposure situation is complicated by the fact that rats have a faster metabolism than humans.
A study made by Nielsen et al. in 2006 on 6 different pesticides showed that as much as 4% of the administered amount of pesticide remained in the epidermis by the end of the experiments after 48 hours and that the relative deposition in the dermis at the same time varied from 0.2% and up to more than 25% (Nielsen JB et al., 2006)
The variation depends of the solubility characteristics of the substances, as the most lipophilic test substances have the greatest relative deposition in the dermis as well as in the epidermis (Nielsen et al 2006).
A study testing chlorpyriphos by applying it to the hands of volunteers and afterwards washing the hands using a standard technique demonstrated that ethanol removed 30% of the chlorpyriphos on the skin at loadings of about 7ug/cm² (Fenske & Lu, 1994). Prewashing with ethanol increased removal efficiency. A 10% isopropanol/distilled water wash removed 43% immediately following exposure and 23% one hour post-exposure with skin loadings of 12ug/cm2 (Fenske & Lu, 1994).
More relevant in relation to normal working conditions is the amount of pesticide deposit in the skin after a working day of 6 hours and also if it is possible to stop the absorption when removing the residue by simple hand wash and the use of a mild soap as is recommended after working with pesticides.
A test substance with a long lag-time showed in the case of methiocarb an increased donor recovery given that the pesticide is removed after 6 hours compared to 48 hours. The difference between the two recoveries could be an estimate of the amount of pesticide that penetrates the skin within the past 42 hours. Since the recovery of methiocarb is 43% after 6 hours and 17% after 48 hours, respectively, the absorption between 6 – 48 hours is 25% of the administered amount. When it comes to dimethoate, which is known to have a lag-time exceeding 20 hours there is no penetration observed after 6 hours. A conclusion could therefore be that a test substance with a long lag-time and a limited affinity towards the more lipophilic compartment has no measurable absorption through the skin and no temporary deposition in the skin after short exposure time (in this study 1/3 of the lag-time). The reason is most likely that it takes more time and also requires a continuous concentration gradient favouring penetration into the skin to overcome the resistance that is due to the high hydrophilicity. Also aspects other than the hydrophilicity are of significance to the skin penetration, which is exemplified by caffeine having a comparable hydrophilicity and a slightly smaller molecular weight and showing clearly different penetration characteristics (Nielsen JB et al., 2006).
11.2. Does skin wash-off remove pesticide residue from the skin surface?
In an in vivo study by Fenske et al. (1998), the efficiency of captan removal from hands by handwashing was evaluated. Removal of 77.8% of the captan transferred to hands was achieved in the group for whom handwashing was done immediately; whereas efficiency was reduced to 68.4% after one hour’s residing on the hands (Fenske et al., 1998). Compared to the results by the same study group on removal of chlorpyriphos, it was demonstrated that removal of this pesticide was significantly lower (43% at time = 0 and 23% at time = 1 hr) (Fenske & Lu, 1994). The variations may be due to the differences in solubility of the two pesticides. Chlorpyriphos is more lipophilic than captan (logPow = 4.96 for chlorpyriphos and logPow = 2.35 for captan)., but also the differences in formulations may have influence on the permeation of the two pesticides, captan being formulated as a wettable powder while the chlorpyriphos formulation used was a liquid concentrate with an emulsifying agent (Fenske & Lu, 1994).
The results found by Nielsen et al. clearly demonstrate that wash-off after 6 hrs removes the predominant part of the administered amount of the test substance and by that reduces the possibility of subsequent skin deposition. At the end of the experiments where the skin has been washed after 6 hrs there is a significantly reduced skin deposition (Nielsen JB et al., 2006). Nielsen et al. also showed that the reduction of skin deposition varied according to solubility. The most hydrophilic test substances (glyphosate and caffeine) demonstrated a reduction in deposition of 80 – 90%, whereas the more lipophilic test substances (malathion and methiocarb) showed a reduction in deposition of only 35% (Nielsen JB et al., 2006).
When the amount of test substance is reduced by wash-off, the consequence is a lower concentration gradient between the skin and the receptor chamber which again as expected causes a decrease in flux. This was confirmed by the reduced receptor recovery seen in the data above. In the most hydrophilic substances a reduction in absorption to 1/3 is seen (expressed by receptor recovery) while the absorption of benzoic acid and methiocarb are reduced to 60-80% of the values seen without wash-off. The penetration coefficients change in agreement with the changes in penetration rate and the amount penetrating.
All together it must be concluded that simple handwashing or wash-off after end of exposure significantly reduces the total absorption of test substances. When it comes to the most hydrophilic substances the reduction is around 67% and for the lipophilic substances the reduction is a little less (Nielsen JB et al., 2006).
This documentation has clear preventive implications regarding wash-off procedures after a working day with dermal exposure to toxic substances.
A study from 2000 showed that after 90 min of pesticide exposure (glyphosate, alachlor, methyl parathion or triflualin) to pig skin it was possible to remove about 50% of the administered dose of pesticide by simple wash using propanol or a soap solution (Campbell et al., 2000). This American study also showed that soap was more efficient in removing pesticides from the skin than e.g. polyethylene glycol (Campbell et al., 2000). The reason for this observation could be that polyethylene glycol has been used as a detergent elsewhere with the purpose of making hydrophilic substances more soluble in water containing solutions and therefore may act as an enhancer to e.g. the absorption of the more hydrophilic pesticide glyphosat. This has been documented in earlier studies which showed the relation between detergents and the absorption of pesticides across skin (Nielsen JB, 2004).
According to current guidelines by OECD dealing with experimental methods in skin penetration, it is prescribed that the amount of pesticide that is not recovered in the donor chamber following exposure should be considered potentially accessible to systemic toxicity (OECD, 2000). This also accounts for the amount of pesticide that is deposited in the skin. The studies from Nielsen et al. show that absorption from the skin to the receptor chamber will continue after end of exposure. For some substances the absorption will even continue more than 24 hours following exposure. Thus, there is no doubt that a part of the residue in the skin will reach the systemic circulation sooner or later, but how much of the residue that actually leaves the skin surface is still not described in general terms but depends on observations from a few test compounds. In an experimental situation this would be the part of the residue that returned to the donor chamber. When testing methiocarb Nielsen et al. have indicated that about 8% of the administered dose was collected after 48 hours in the groups where the cells were washed after 6 hours. Since it is no more than 15% of the administered methiocarb dose that is found in the skin after 48 hours the 8% constitutes 1/3 of the amount of residue that would have been deposited in the skin if there had been no backward diffusion to the donor chamber. These data indicate that a considerable part of the residue that is temporary deposit in the skin returns to the donor chamber. Data representing the lipophilic substances show that there is a slight overestimation when approximating a reliable absorbable amount of substance by counting all the substance that is not retrieved in the donor chamber as absorbed. The overestimation, however, is not large and for the lipophilic substances the procedure used so far seems of practical use (Nielsen JB et al., 2006).
12 Prevention of dermal absorption
- 12.1 Dermal exposure to pesticides
- 12.2 Personal protective gear
- 12.3 Penetration of benzoic acid through gloves
- 12.4 Penetration of pesticides through gloves
- 12.5 Types of gloves
- 12.6 The use and re-use of gloves
Risk is defined as the possibility that a worker or the environment may be harmed in a particular process. The toxicity or hazard of the pesticide cannot be altered, but the risk can be handled through use of appropriate protective gear and proper management and application.
12.1 Dermal exposure to pesticides
Exposure to pesticides is seen through working with different plants, fruits, crops, flowers etc. The degrees of exposure vary from the frequent, specific use in limited areas like for example greenhouses to the less frequent use in large outdoor facilities. The different intensities may be exemplified by the fact that a study from 2001 showed that a typical Danish ornamental greenhouse is using pesticides or growth retardants more than 50 times a year in contrast to conventional Danish farmland only being treated 2 to 3 times a year (Andersen & Nielsen JB, 2001).
Work-related exposure to pesticides may happen throughout the production, mixing, and loading of strong commercial products, distribution and management of diluted pesticides, and through re-entry activities. The different jobs are characterized by causing a combination of instant exposure (splash etc.) to concentrated commercial products and continued exposures to lower concentrations by handling stems, leaves or soil after pesticide treatment. Studies have found that workers performing the farm task of thinning are more exposed to pesticides then e.g. workers harvesting or pruning (de Cock et al., 1998b;de Cock et al., 1998a;Simcox et al., 1999). One group found a higher level of pesticides in the house and vehicle dust of the workers thinning and a higher pesticide metabolite concentration was found in their children’s urine as an evidence of the take-home pesticide pathway (Coronado et al., 2004;Coronado et al., 2006).
Previous investigations have examined workplace protective practices of field- and greenhouse workers. Their use of gloves, long-sleeved shirts, and long pants (Vela-Acosta et al., 2002;Sjelborg P et al., 2008) but only limited investigations have assessed pesticide exposure among these groups of workers (Simcox et al., 1999;Fenske, 2005;Strong et al., 2004) and the prevention of work-to-home transmission of pesticides as described in a review by Fenske 2006 (Fenske et al., 2006).
12.1.1 Mixing and loading
When the sales products are delivered to the user, the concentration of the product is significantly greater than the concentration used for treatment of the plants. When mixing and loading the pesticide there is a present risk of being exposed to the concentrated pesticide. Often the kind of exposure is an instant exposure e.g. splashes. If a worker has areas of skin not covered by protective gear (gloves, etc.) an uncontrolled splash may cause unintended exposure to the skin - often the hands - and may cause a significant risk of toxicity. Exposure of the hands can be an important contributor to the total exposure of the skin and has been shown to account for between 50 and 90% of the total body exposure (Abbott et al., 1987;Archibald et al., 1995;Karr et al., 1992).
12.1.2 Distribution
The distribution of pesticides may engage automatic spraying, spraying from person-driven vehicles, hand-carried spraying, or watering systems. Distribution involves varying exposure times to varying concentrations of pesticides with varying toxicity. Though, the concentrations will not be expected to cause acute toxicity after an accidental short-term exposure, it is still important to focus on avoiding long-term dermal contact with pesticides. This means a necessity to use gloves at all times during spraying operations, which again asks for a certain degree of comfort if good compliance should be attained. When distributing the pesticides a considerably part of the body may be exposed besides the hands - even the respiratory system may be involved. In these cases, gloves will only be part of the preventive attempt to diminish body dose.
12.1.3 Re-entry
Exposure by re-entry happens when workers enter areas recently treated with pesticides. Leaves, stems and soil may have pesticide depositions and workers may be dermally exposed to these residues. How long the pesticide remains on the plant or soil and therefore is an occupational risk depends on chemical stability, stability against sunlight, and metabolism of the active ingredient. Compared to mixing, loading and distribution the concentrations at re-entry are lower but at times workers are exposed to the pesticides for an entire working day. The crop handling may require some dexterity and may be difficult to obtain with all glove types and materials. Moreover, the workers may not be aware that they are actually exposed. Therefore it is important to define re-entry intervals that allow the pesticide to wash off or degrade before the crop is handled again and also to use gloves whenever handling plants recently treated with pesticides.
12.2 Personal protective gear
Most important when managing pesticides, whether it is in the process of mixing, loading or distributing or it is handling the crop after pesticide treatment, is the use of protective gloves. The main problem that influences the choice and ultimately the use of gloves in a work situation is the discomfort of the gloves, but also the resistance, durability and penetration characteristics of the gloves play an important role in the safety procedure. Often comfort and resistance are not easy to combine in one type of gloves and if so the gloves are most likely very expensive and therefore not a priority especially in the developing countries. Preferably, people handling pesticides should always use the glove giving maximum protection. Since this glove is often not the most comfortable choice, a problem has arisen. To try and solve this problem it is important to have specific knowledge on the pesticides used and the degree of exposure the worker is subjected to.
12.2.1 Penetration characteristics of gloves
Penetration characteristics may be described as breakthrough time and penetration rate. To judge the quality of the glove the breakthrough time is one of the most used parameters. This parameter describes the time from the beginning of the exposure until the pesticide appears on the inside of the glove. The time range is from 15 min to more than 24 hours for different pesticides through different types of glove materials (Creely & Cherrie, 2001;Ehntholt et al., 1990;Guo et al., 2001;Krzeminska & Szczecinska, 2001;Moody & Ritter, 1990;Moody & Nadeau, 1994;Purdham et al., 2001;Schwope et al., 1992;Silkowski et al., 1984). Further, the resistance against penetration through a specific glove material will depend on pesticide materials (Creely & Cherrie, 2001;Ehntholt et al., 1990;Guo et al., 2001;Krzeminska & Szczecinska, 2001;Moody & Ritter, 1990;Moody & Nadeau, 1994;Purdham et al., 2001;Schwope et al., 1992;Silkowski et al., 1984)as well as formulation (Ehntholt et al., 1990;Harville & Que Hee, 1989;Moody & Nadeau, 1994;Nielsen & Andersen, 2001;Purdham et al., 2001;Silkowski et al., 1984).
The reasons why gloves fail to provide effective protection can be divided into four categories:
- Misuse
- Physical damage
- Degradation
- Permeation
In many situations, it will be a combination of these four reasons that leaves the user exposed to the chemical, often without realizing it (Packham, 2006).
The breakthrough time will not say anything about the amount of pesticide absorbed into the glove or the penetration rate after exposure, but it is still an important measurement since, as illustrated in Figure 22, two glove types may have identical penetration after a 6 hour period but very different breakthrough time and penetration rate. Thus, if the work time were 4 hours, glove 2 would definitely be preferred.
Essential knowledge when using disposable gloves is:
- Information on breakthrough time
- Know when to change gloves to avoid crossing the limit for breakthrough time
- If the gloves are used after breakthrough it is worth knowing the penetration rate
Information on penetration rate and reservoir within the glove material is of importance if gloves are used several times. (Nielsen JB, 2005b).
Figure 22: Penetration of two pesticides (1 and 2) through gloves. Penetration of 1 and 2 is identical, but the breakthrough times and penetration rate are different. (Nielsen JB, 2005b)
Another important parameter that may influence skin exposure is physical and/or chemical degradation of the gloves worn for personal protection. The influence of hand movements on the integrity of the gloves is often not considered when studying glove permeation/penetration. In a recent study a robotic hand, simulating normal hand motions, was used to assess the influence of hand movement on the permeation through nitrile rubber gloves. Even though hand movement did not appear to significantly affect the permeation of captan through the gloves, the movements did influence physical and/or chemical degradation, resulting in glove failure (Phalen & Hee, 2008). Thus, future research should continue to investigate the influence of hand movement and additional work factors on the permeation, penetration, and physical integrity of protective gloves.
12.3 Penetration of benzoic acid through gloves
Benzoic acid has been used as a model compound to demonstrate the effect of different glove materials used in an identical experimental set-up. Further, these studies allow important observations on the influence of dose on glove penetration. Eventually, these experiments demonstrate the significant fraction of the applied dose residing in the gloves when exposure is terminated, which is of considerable importance when discussing gloves intended for repeated use.
From figures 23 and 24 and Table 7 and Table 8 it is evident that gloves significantly reduces total penetration, but the effect depends on glove material, dose of chemical applied on the glove, and the influence on lag-time is limited. At the lowest dose, nitril offers very good protection as the maximal flux is reduced 25-fold as contrasted by the 50% reduction in penetration offered by the latex material (Table 7). The relative protection is drastically reduced at the higher dose (Table 8) and illustrates that a glove suitable for low dose exposure, e,g, during re-entry situations, may not be ideal for higher doses, e.g. during mixing and loading were undiluted pesticides are used. One explanation is that the glove functions as a temporary deposit, but with a limited capacity. Thus, substatntial amounts of benzoic acid are recovered from the glove material at the end of exposure, more so in nitril gloves (Tables 7 and 8). In experiments with gloves-only, comparable amounts of benzoic acid was deposited in the gloves as in experiments with underlying skin (Tables 7 and 9). Glove recovery accounted for 40% (latex) and 74% (nitril) of the applied dose of benzoic acid. In experiments with tebuconalzol, which is less hydrophilic and has a higher molecular weight, approximately 18% of the applied dose was recovred in latex gloves, which illustrates that glove deposition may vary depending on the pesticide as well as the dose applied.
Table 7. Dermal penetration characteristics following topical application of benzoic acid (4 mg/mL)
| Control | Latex | Nitril | |
| Cotton swabs (µg) | 15.6 | ||
| Donor wash (µg) | 11.6 | 11.3 | 12.9 |
| Donor recovery (µg) | 27.2 | 11.3 | 12.9 |
| Glove recovery (µg) | 116.3 | 271.1 | |
| Between glove and skin (µg) | 1.4 | 2.6 | |
| Epidermis (µg) | 1.3 | 4.2 | 3.2 |
| Dermis (µg) | 8.2 | 8.7 | 6.1 |
| Skin recovery (µg) | 9.5 | 12.9 | 9.3 |
| Receptor recovery (µg) | 398.4 | 239.5 | 42.1 |
| Experimental recovery (µg) | 435.0 | 381.3 | 338.1 |
| Experimental recovery (pct) | 102.6 | 89.9 | 79.7 |
| Maximal flux (µg/hr/cm2) | 13.1 | 6.6 | 0.5 |
| Lag-time (hr) | 2.5 | 3.1 | 7.0 |
Based on the maximal flux, an apparent Kρ may be calculated for the
penetration of benzoic acid in controls, and is found to be 33 µm/hr.
Table 8. Dermal penetration characteristics following topical application of benzoic acid (40 mg/mL)
| Control | Latex | Nitril | |
| Cotton swabs (µg) | 89.6 | ||
| Donor wash (µg) | 10.0 | 83.6 | 150.8 |
| Donor recovery (µg) | 99.6 | 83.6 | 150.8 |
| Glove recovery (µg) | 320.8 | 1225.6 | |
| Between glove and skin (µg) | 5.2 | 14.4 | |
| Epidermis (µg) | 8.3 | 10.8 | 17.1 |
| Dermis (µg) | 147.0 | 137.8 | 165.6 |
| Skin recovery (µg) | 155.4 | 148.6 | 182.8 |
| Receptor recovery (µg) | 4493.5 | 3815.6 | 2712.9 |
| Experimental recovery (µg) | 4748.5 | 4373.8 | 4286.4 |
| Experimental recovery (pct) | 112.0 | 103.6 | 101.1 |
| Maximal flux (µg/hr/cm2) | 166.4 | 104.0 | 47.4 |
| Lag-time (hr) | 1.2 | 2.4 | 2.4 |
Based on the maximal flux, an apparent Kρ may be calculated for the penetration of benzoic acid in controls, and is found to be 42 µm/hr.
Table 9. Penetration characteristics following application of benzoic acid (4 mg/mL) to static diffusion cells mounted with gloves only.
| Latex | Nitril | ||
| Donor recovery (µg) | 7.9 | 16.9 | |
| Glove recovery (µg) | 132.8 | 252.8 | |
| Glove recovery (pct) | 40.0 | 74.0 | |
| Receptor recovery (µg) | 190.1 | 72.6 | |
| Experimental recovery (µg) | 330.7 | 342.3 | |
| Experimental recovery (pct) | 78.0 | 80.7 | |
| Maximal flux (µg/hr/cm2) | 11.2 | 1.6 | |
| Lag-time (hr) | 0 | 1.8 |
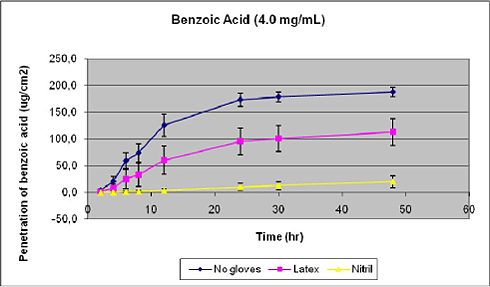
Figure 23. Influence of gloves on percutaneous penetration of benzoic acid. Glove material was mounted on top of the skin in the static diffusion cells. Results are given as mean + SEM (n=6).
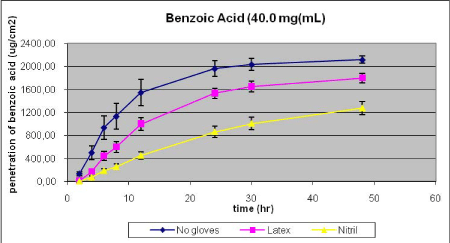
Figure 24. Influence of gloves on percutaneous penetration of benzoic acid. Gove material was mounted on top of the skin in the static diffusion cells. Results are given as mean + SEM (n=6).
Another interesting observation was that the observed lag-times in the experiments including human skin were not increased very much, which is in agreement with the low break-through times observed in the experiment with gloves only (Table 9). Lag-time for tebuconazol through latex gloves was comparable to the lag-time for benzoic acid through latex material (data not shown).
The results indicate that the amount of chemical residing between glove and skin is limited, irrespectively of glove material and dose (Tables 7 and 8).
The conclusions based on these experiments are that nitril gloves appear to offer a better protection than latex gloves against penetration of benzoic acid, but that the efficacy of the gloves is significantly reduced at higher doses. Single-use gloves should not be used for extended periods as lag-times are limited for both glove materials and an apparent accumulation occurs in the glove materials, which may reach saturation.
The observed accumulation of chemical within the glove material calls for further investigation as this may have profound influence on the guidance on use of gloves intended for repeated use. If a significant fraction of chemical is accumulated in the glove everytime it is used, the glove may become saturated and loose its efficacy for protection – without the user being aware of it.
Generally, the published information on penetration of pesticides through gloves is scattered and based on different experimental models mostly focusing on break-through times. Below we have summarized the available information on penetration of pesticides through gloves.
12.4 Penetration of pesticides through gloves
12.4.1 Carbamates
Penetration characteristics for these insecticides through gloves are studied for carbaryl, methomyl, sulfallate, methiocarb and pirimicarb (DuPont Co Protective Apparel Fabrics of TYVEK, 1993;Keith LH et al., 1000;Moody & Ritter, 1990;Nielsen & Andersen, 2001;Raheel & Dai, 1997;Raheel & Dai, 2002). The breakthrough times in all studies exceed 8 hours for PVC (polyvinyl chloride), nitrile butyl rubber, natural rubber and Neoprene® gloves (Moody & Ritter, 1990). Also gloves made of butyl rubber, nitrile or Viton® showed breakthrough times against sulfallate exceeding 8 hours, whereas Neoprene® had a breakthrough time of 4 hours only (Keith LH et al., 1000). Conclusions have been drawn that nitrile, butyl and Viton® have a higher resistance towards chemicals and penetration than latex/natural rubber or PVC gloves (Raheel & Dai, 1997;Raheel & Dai, 2002). Polyethylene gloves were penetrated by methomyl within 15 min (DuPont Co Protective Apparel Fabrics of TYVEK, 1993). Comparing the penetration of pirimicarb and methiocarb through latex or nitrile gloves has revealed that while latex reduces penetration of both carbamates by 50%, nitrile offers more than 90% protection against pirimicarb during an 18-hours test period (Nielsen & Andersen, 2001). In general, gloves made of butyl rubber, nitrile and Viton® proved to offer the best protection against carbamates.
12.4.2 Pyrethroids
Cypermethrin, permethin and tefluthrin were tested in this group of insecticides. Tefluthrin had a breakthrough time above 24 hours through nitrile, Neoprene and barrier laminate (SilverShieldTM trademark of Siebe North, Inc.)(Guo et al., 2001), but an increasing amount of pesticides remained attached to or absorbed into the glove made of barrier laminate by increased exposure time. Therefore barrier laminate gloves should not be re-used since they cannot be cleaned. Butyl rubber had a breakthrough time above 8 hours for cypermethrin (Krzeminska & Szczecinska, 2001) . Comparing the potential exposure (on the outside of the gloves) with the actual exposure (on the inside of the gloves) of permethin and two nitrile and one PVC glove it showed protection factors of 470, 200 and 96, respectively. This means that during a 20 min work period the penetration of permethin through gloves worn by volunteers was reduced to between 0.2% (nitrile) and 1% (PVC) of the potential exposure (Creely & Cherrie, 2001).
In general, the gloves made of nitrile, Neoprene, barrier laminate, butyl rubber and PVC showed good protection against penetration of this group of pyrethroids, when taking into account that barrier laminate is suggested to be used only as a single-use material (Guo et al., 2002).
12.4.3 Aryloxyalcanoic acids
2,4-D and MCPA were in this group of herbicides studies in different formulations. MCPA was tested in two undiluted formulations, one salt and one ester on four types of gloves (Purdham et al., 2001). The salt formulation showed no permeation in 24 hours but the ester formulation penetrated all four types of gloves within 24 hours - latex and Neoprene in 15 min and nitrile in 24 hours. The nitrile glove had the longest breakthrough time and the slowest penetration rate where Neoprene had a penetration rate that exceeded the other gloves by 3- to 7-fold (Purdham et al., 2001).
2,4-D demonstrated a breakthrough time above 8 hours in nitrile, butyl rubber, PVC, latex and Neoprene gloves (Krzeminska & Szczecinska, 2001;Moody & Ritter, 1990), although one study points out that Neoprene gloves were permeated much more than nitrile gloves (Harville & Que Hee, 1989). Several studies show that glove penetration varies between nitrile gloves and varies between different pesticide formulations and draws attention to the problems when extrapolating characteristics between pesticide formulations and even between gloves made of identical materials (Harville & Que Hee, 1989;Krzeminska & Szczecinska, 2001;Moody & Nadeau, 1994).
In general, the glove materials show fine protection against these herbicides, although gloves made of nitrile offered the longest breakthrough time and the lowest penetration rate after breakthrough.
12.4.4 Organochlorines
This group of insecticides have been analysed in two different studies (Ehntholt et al., 1990;Moody & Nadeau, 1994). Moody et al. demonstrated that DDT had an insignificant penetration through nitrile butyl rubber gloves during 24 hours (Moody & Nadeau, 1994), while Ehntholt et al. showed a breakthrough time of only 15 min for endosulfan (endosulfan 34% in xylene 57%) through several glove materials (butyl rubber, latex, polyethylene, PVC, SilverShield, nitrile) except for Neoprene which had a 30 – 60 min breakthrough time. The quantity of endosulfan penetrating the gloves during 8 hours demonstrated that 10 to 100 times less endosulfan penetrated gloves made of SilverShield and nitrile than other glove materials (Ehntholt et al., 1990).The conclusion was that gloves made of nitrile and SilverShield were most resistant to penetration and latex and polyethylene gloves the least resistant.
In general, the literature is scarce but suggests that breakthrough times for these insecticides are short in almost all types of materials, but SilverShield and nitrile gloves are the most resistant.
12.4.5 Organophosphates
Data on penetration characteristics through gloves of 8 insecticides in this group were identified. The pesticides were: azinphos-methyl, diazinon, ethyl-parathion, malathion, methyl-parathion, monocrotophos, tricresyl phosphate and terbufos (Ansell Protective Products, 1998;Ehntholt et al., 1990;Guo et al., 2002;Keeble et al., 1993;Keeble et al., 1996;Moody & Nadeau, 1994;Safety 4, 1993). Nitrile, Neoprene and SilverShield had a breakthrough time of more than 24 hours, although increasing exposure time showed that an increasing amount of pesticide was absorbed into the Neoprene gloves (Guo et al., 2001). Polyethylene gloves as well as cotton gloves reduced the penetration of azinphos-methyl and malathion in an in vitro study, but did not avoid penetration during a 4-hour observation period (Keeble et al., 1993;Keeble et al., 1996). When testing 4HTM laminate gloves from Safety4 a/s the breakthrough time increased to above 4 hours for malathion and glyphosate (Round Up®) (Safety 4, 1993). Gloves made of polyester or nylon showed no significant protection against azinphos-methyl (Keeble et al., 1993). The pesticide diazinon was tested in a sales formulation which demonstrated no significant penetration through nitrile butyl rubber over a 24-hour period. In table 10 different data on breakthrough times and relative permeation are summarized by Nielsen JB (Nielsen, 2005b).
Table 10. Breakthrough times and relative permeation of three organophosphates through seven glove materials
| Ethyl parathion | Methyl parathion | Azinphos-methyl | Monocroto phos |
Tricresyl phosphate | ||||||
| BT* (h) | RP* | BT (h) | RP | BT (h) | RP | BT (h) | RP | BT (h) | RP | |
| Natural rubber | 1-2 | + | <½ | +++ | <3 | + | 4-6 | ++ | <1 | + |
| Nitrile | 6-8 | 0 | 1-2 | ++ | >8 | 0 | 1-2 | ++ | >6 | 0 |
| Neoprene® | 3-4 | + | 1-2 | +++ | >8 | 0 | 3-4 | + | <½ | +++ |
| Polyethylene | <½ | +++ | <½ | +++ | ||||||
| PVC | 4-6 | + | <½ | +++ | 4-5 | ++ | <½ | ++ | >6 | + |
| Butyl rubber | >8 | 0 | 3-4 | ++ | ||||||
| SilverShield® | 4-6 | + | <½ | ++ | <½ | + | ||||
BT – breakthrough time; RP. – relative permeation
From this table it is shown that none of the glove materials perform very well against monocrotophos, though Neoprene and SilverShield have the lowest relative penetration. Similarly, methyl parathion penetrates most glove materials very fast and has a large quantitative penetration. Against the permeation of ethyl parathion, nitrile, SilverShield, PVC and butyl rubber demonstrated breakthrough times around 4-8 hours with butyl rubber and nitrile as the best material when relative permeation was taken into account. Not many of the tested glove materials demonstrated good penetration characteristics against tricresyl phosphate except for PVC and nitrile with a breakthrough time above 6 hours (Table 22.1).
All the current data illustrate the variety in penetration characteristics present even in the same group of pesticides. In general nitrile, butyl rubber and SilverShield were the most resistant towards penetration of the organophosphates that were tested. Latex, polyethylene and cotton were the least resistant. However, none of the glove materials demonstrated very good protection against all the pesticides studied.
12.5 Types of gloves
12.5.1 Polyethylene gloves
Different studies for the permeation of organophosphates, carbamates and organochlorines through polyethylene gloves were summarized and all data showed a very short breakthrough time of less than 15 min and a large penetration rate (DuPont Co Protective Apparel Fabrics of TYVEK, 1993;Ehntholt et al., 1990;Schwope et al., 1992)). Consequently, these gloves cannot be recommended as protection against pesticide exposure.
12.5.2 PVC
Only a few of the studied pesticides: 2,4-D, tricresyl phosphate and pentachlorophenol had breakthrough times above 6 hours (Ansell Protective Products, 1998;Moody & Ritter, 1990;Silkowski et al., 1984)). All other studied pesticides had a low breakthrough time and/or a high penetration rate and PVC generally offered poor protection against these pesticides (Creely & Cherrie, 2001;Ehntholt et al., 1990;Raheel & Dai, 1997;Raheel & Dai, 2002;Schwope et al., 1992).
12.5.3 Neoprene
Neoprene demonstrated good protection against some pesticides like: a carbamate, a triazine, several organophosphates, a pyrethroid and two aryloxyalcanoic acids (Ansell Protective Products, 1998;Cessna & Grover, 2002;Guo et al., 2001;Harville & Que Hee, 1989;Moody & Ritter, 1990;Purdham et al., 2001;Raheel & Dai, 1997;Raheel & Dai, 2002). But against organophosphates like ethyl-parathion and methyl-parathion, monocrotophos, endosulfan and pentachlorophenol the protection was poor.
12.5.4 Latex, natural rubber
Latex gloves have only shown breakthrough times of more than 8 hours in one study by Moody and Ritter when they studied carbamate carbonyl and 2,4-D. In this study the carbamate carbonyl was used as a dry powder, which may have influenced the penetration characteristics. All other data on this material for most pesticide groups show breakthrough times around 30 min and large penetration rates after that. A Danish field study on greenhouse workers demonstrated a protection by latex gloves of 93%, a breakthrough time for the fungicide Amistar® of less than 2 hours and an exposure of 3% when removing the gloves afterwards (Kirknel E & Sjelborg P, 2003).
If alternatives exist latex/natural rubber gloves should not be used as protective gear against pesticides.
12.5.5 Nitrile
All but one study demonstrate that nitrile gloves offer good protection against the pesticides tested (Ansell Protective Products, 1998;Creely & Cherrie, 2001;Ehntholt et al., 1990;Guo et al., 2001;Harville & Que Hee, 1989;Kirknel E & Sjelborg P, 2003;Moody & Ritter, 1990;Moody & Nadeau, 1994;Nielsen & Andersen, 2001;Purdham et al., 2001;Raheel & Dai, 1997;Schwope et al., 1992;Silkowski et al., 1984)). In most studies the breakthrough time was above 8 hours although one study showed breakthrough times for methyl-parathion, endosulfan and monocrotophos through nitrile glove of less than 30 min. In this study the conclusion was still that nitrile showed good protection to these pesticides compared with other gloves (Ehntholt et al., 1990). Kirknel et al. demonstrated a protection of 97% but also in this study exposure of the bare hands after removing the gloves was observed. This study group also demonstrated tears in the gloves at the end of experiments. In 17 out of 114 latex gloves this was the case whereas only 6 nitrile gloves were damaged (Kirknel E & Sjelborg P, 2003).
12,5.6 SilverShield/Laminate
Five pesticides have been tested against laminate glove material. The material showed relatively good protection against the pesticides: terbufos, tefluthrin, pentachlorophenol, 2,4-D and ethyl-parathion. (Ehntholt et al., 1990;Guo et al., 2001;Guo et al., 2002;Harville & Que Hee, 1989;Schwope et al., 1992). In one study monocrotophos, endosulfan and methyl-parathion penetrated the SilverShield gloves in 30 min (Ehntholt et al., 1990) and Guo et al. concluded that the laminate glove was a single-use glove given that the material could not be cleaned after use, but no penetration of the remaining pesticide was demonstrated (Guo et al., 2002).
12.5.7 Cotton liners
To increase the comfort of wearing gloves during work, cotton knit gloves (liners) worn under nitrile chemical-resistant gloves (CRG) have been tested on greenhouse workers. The workers felt more comfortable with the liners underneath their work gloves. However, contamination of the liners were demonstrated even though the degree of contamination was significantly lower than on the CRG (Stone et al., 2005). These results support the Environmental Protection Agency's recommendation that liners should be disposable (US Environmental Protection Agency, 2004).
12.6 The use and re-use of gloves
When using gloves as protective equipment against pesticide exposure it is important to know how to store and keep the gloves before and after use - if the gloves are reusable. As most people know from experience the structural integrity of glove materials decreases with time, but information on how the penetration characteristics change is insufficient and often information on storage conditions of the gloves is not available for the user. Raheel and Dai have demonstrated that only 10 days storage at -3°C changes the flexibility (nitrile gloves) as well as the resistance (Neoprene, nitrile and latex gloves) (Raheel & Dai, 2002).
12.6.1 Reusable gloves
Reusable gloves are often used around pesticides and other toxic substances. Repeated use without effective decontamination may result in secondary exposure. A study testing neoprene, Guardian butyl rubber, and nitrile synthetic rubber gloves against toluene and acetone used thermal decontamination and found this method to be effective in removing the solvents without significant degradation of the glove materials (Gao et al., 2005). Although the manufacturers of the gloves describe how to clean them before reuse, one study has shown that residues of pesticides still remain inside the gloves after cleaning (Guo et al., 2002). The same study suggested that gloves made of barrier laminate should not be reused since the material cannot be cleaned (Guo et al., 2002). Moody and Nadeau demonstrated a reservoir effect of 2,4-D, DDT and diazinon within the glove when a considerable amount of the substances could be extracted from the gloves after cleaning (Moody & Nadeau, 1994). As mentioned earlier, however, the study did not demonstrate if the pesticides were available for later penetration and absorption.
When protective gloves are taken off, exposure can be even more substantial and this is often underestimated (Garrod et al., 2001;Edwards et al., 2007). It is difficult to avoid touching the exterior of the gloves and a recent study has also shown that the inside of the gloves is often contaminated just as the outside (Creely & Cherrie, 2001;Garrod et al., 2001;Machera et al., 2003). If the hands are contaminated inside the gloves an occlusive environment and an intimate contact with the hazardous substance occur. Since occlusion has proven to increase skin penetration it is of great importance to avoid chemicals inside a glove (Wester & Maibach, 1983). More research on this issue is required to obtain further knowledge on how the occlusion affects the penetration. A few studies have studied gloves on top of a skin membrane (Keeble et al., 1996;Nielsen & Andersen, 2001), but data describing enhanced penetration through human skin are not further elaborated.
Kirknel and Sjelborg found that as much as 50% of the total pesticide exposure occurs when the employees change their gloves during work (Kirknel E & Sjelborg P, 2003). Because of the interindividual variation of exposure in this procedure, is it important that the users are aware of the risks and well educated to avoid unnecessary contamination. Rawson et al. showed that without training 9 out of 10 volunteers had internal contamination of their gloves when they reused them. However, if they were trained this was reduced to 1 out of 10. Although single-use gloves may generally reduce the potential for internal contamination, this study demonstrated that 3 out of 10 volunteers were contaminated due to leaking or faulty gloves. Wearing gloves which are internally contaminated can lead to increased systemic absorption due to increased area of contact and reduced skin barrier properties, and repeated skin contact with low volatility chemicals can give higher than expected exposure if evaporation of the carrier occurs and the concentration in contact with the skin increases (Rawson et al., 2005).
From practical experience it is also known that disposable gloves are sometimes reused. Since the integrity of the gloves changes and the gloves might be contaminated on the inside after prior use, the exposal situation becomes even more essential. When an employee actually falsely believes that the gloves protect against chemical exposure, the situation is worse than if a worker despite understanding the danger chooses not to use gloves since he is probably aware of the potential risk and therefore takes better precautions. A situation with an employee feeling safe, and acting as if protected, is unacceptable.
Because of the problems mentioned above, the important elements in the preventive strategy to reduce exposure must be to educate, train and supervise the users better. If the problem about reusing gloves (reusable and disposable) is not given further research attention the advice will be to only use disposable gloves and dispose them afterwards.
By using gloves and other personal protective equipments the hope is to change unacceptable exposure into an acceptable risk.
13 Conclusions and perspectives
13.1 Regulatory aspects
- Knowledge on percutaneous penetration may originate from in vitro as well as in vivo experiments and from observational studies in humans during and after exposure. Quantitative differences will exist, and is primarily due to the inherent heterogeneity between humans and therefore also between donors for the in vitro experiments, but qualitative comparisons across different approved methods appear quite solid.
- Results based on in vitro experiments carried out on human skin according to the present OECD guidelines have repeatedly demonstrated good agreement with experience from human exposure situations.
- Results from in vitro experiment on human skin following OECD guidelines are normally seen as more predictive than animal experiments in mice or rats.
- Evaluation of dermal penetration of chemicals with known skin metabolism requires special attention.
- The OECD guidelines state that the amount of substance not found in the donor chamber should be considered absorbed and therefore potentially available in the systemic circulation. This also accounts for the amount of substance deposited in the skin. Experiments demonstrate that absorption of chemicals temporarily deposited in the skin continues for up to 24 h after exposure has ended. Studies have agreed on this statement but this report also indicates that a fraction of the substance temporarily deposited in the skin returns to the donor chamber.
- For lipophilic substances, the overestimated absorption is however not considered big and should probably not influence the present conservative estimates used by regulatory agencies.
- Conclusions regarding more hydrophilic chemicals still need some elaboration on the hydrophilic part of the spectrum.
- Temporary skin deposition will potentially underestimate the true absorption if assessed in blood or urine immediately following exposure.
- Percutaneous penetration is strongly influenced by the solubility (e.g. logPow) as well as the molecular size of the penetrant. Several mathematical models to predict percutaneous penetration based on large databases on penetration of +100 chemicals exist.
- For chemicals with logPow values between -2 and 2, there appear to be a positive correlation between logPow and Kρ (penetration rate).
- If a chemical does not have specified and reactive chemical groups or high affinity for proteins in the skin, these models will within a reasonable broad spectrum of solubilities deliver valid predictions on expected skin penetration.
- Use of mathematic models for regulatory purposes requires significant experience and knowledge, and several studies have demonstrated that many chemicals without known reasons do not fit the present mathematical models.
- Washing the skin after exposure has proved to decrease the amount of substance available for later penetration significantly. The deposition of hydrophilic substances is most affected and the absorption is reduced to one third.
- There is solid evidence that hand wash following skin exposure to pesticides will reduce systemic exposure.
- This observation has a clear preventive perspective in relation to training and personal hygiene for pesticide users.
- Slightly damaged skin results in a generally increased penetration rate of all substances.
- The effect is most significantly in the hydrophilic substances.
- This observation is important given that a significant part of the work force suffers from more or less chronic skin problems and therefore has a compromised skin barrier.
- Regulatory agencies should consider how this information can be included in their rule setting, regulatory policies, as well as their guidance given to users of pesticides.
- Gloves have proven to decrease the penetration and absorption rate of pesticides, but breakthrough-times differ between glove materials and some glove materials offer better protection against specific pesticides than others.
- By short-term exposure (< 2 hours) to low concentrations of pesticides (not mixing and loading) latex gloves will offer slightly less but fair protection compared to nitril gloves.
- Repeated use of disposable gloves not intended for re-use should be avoided.
- Repeated use of gloves produced for that purpose requires attention regarding training and personal hygiene.
- Penetration through gloves depends on amount and concentration of the pesticide and accumulation within glove material may occur.
- During use of sales products with high concentrations of pesticides (e.g. during mixing and loading), the protective efficacy of disposable gloves made of latex or nitril will be severely reduced and other or more effective material is recommended.
- If accumulation of pesticide in the glove material is suspected, this may reduce the re-use of the gloves significantly.
13.2 Research aspects
- A future perspective is the investigation of the reservoir effect in relation to solubility and molecular weight. This include:
- a thorough analysis of the amount of substance persisting in the skin after end of exposure
- the quantification of the fraction of substance that continuously penetrates to the systemic circulation after end exposure
- the assessment of the fraction that may be removed from the skin following different cleansing procedures
- When studying dermal penetration it has been custom to focus on the active ingredient and not the commercial products. This report has described the influence of different detergents present in formulated products on penetration rate and lag-time.
- Therefore, there is a need to focus research on influence of detergents on penetration characteristics of sales products.
- Recognizing that the evidence for extrapolation between products containing the same active ingredient but different detergents may not be adequate, will require a continued focus on development of a best practice on reevaluation of already approved pesticides is needed.
- Pesticides are often used in combination, and research has demonstrated increased as well as a decreased penetration rates depending on the substances.
- The studying of pesticides in combination is therefore an area that needs continued attention.
- More recent experimental evidence demonstrates accumulation of chemicals in glove materials, which have clear implications for gloves used repeatedly.
- This area should receive specific attention as the exposure situations involving use of non-disposable gloves often involves use of sales formulations of pesticides (production, mixing, loading), where concentrations of pesticides handled are orders of magnitude higher than used during application.
Reference list
Abbott IM, Bonsall JL, Chester G, Hart TB, & Turnbull GJ (1987). Worker Exposure to A Herbicide Applied with Ground Sprayers in the United-Kingdom. American Industrial Hygiene Association Journal 48, 167-175.
Akomeah F, Nazir T, Martin GP, & Brown MB (2004). Effect of heat on the percutaneous absorption and skin retention of three model penetrants. European Journal of Pharmaceutical Sciences 21, 337-345.
Akomeah FK, Martin GP, & Brown MB (2007). Variability in human skin permeability in vitro: Comparing penetrants with different physicochemical properties. Journal of Pharmaceutical Sciences 96, 824-834.
Akomeah FK, Martin GP, Muddle AG, & Brown MB (2008). Effect of abrasion induced by a rotating brush on the skin permeation of solutes with varying physicochemical properties. European Journal of Pharmaceutics and Biopharmaceutics 68, 724-734.
Alberti I, Kalia YN, Naik A, Bonny J, & Guy RH (2001a). Effect of ethanol and isopropyl myristate on the availability of topical terbinafine in human stratum corneum, in vivo. Int J Pharm 219, 11-19.
Alberti I, Kalia YN, Naik A, Bonny JD, & Guy RH (2001b). In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release 71, 319-327.
Andersen HR & Nielsen JB. A Danish survey on use of pesticides and gloves in ornamental greenhouses. Unpublished report. 2001.
Ref Type: Report
Anderson C, Andersson T, & Molander M (1991). Ethanol absorption across human skin measured by in vivo microdialysis technique. Acta Derm Venereol 71, 389-393.
Ansell Protective Products (1998). Chemical resistance guide, 6 ed. Ansell Protective Products, Coshocton, OH.
Archibald BA, Solomon KR, & Stephenson GR (1995). Estimation of Pesticide Exposure to Greenhouse Applicators Using Video Imaging and Other Assessment Techniques. American Industrial Hygiene Association Journal 56, 226-235.
Atrux-Tallau N, Pirot F, Falson F, Roberts MS, & Maibach HI (2007). Qualitative and quantitative comparison of heat separated epidermis and dermatomed skin in percutaneous absorption studies. Archives of Dermatological Research 299, 507-511.
Bashir SJ, Chew AL, Anigbogu A, Dreher F, & Maibach HI (2001). Physical and physiological effects of stratum corneum tape stripping. Skin Res Technol 7, 40-48.
Baynes RE, Brooks JD, Mumtaz M, & Riviere JE (2002). Effect of chemical interactions in pentachlorophenol mixtures on skin and membrane transport. Toxicol Sci 69, 295-305.
Baynes RE & Riviere JE (1998). Influence of inert ingredients in pesticide formulations on dermal absorption of carbaryl. American Journal of Veterinary Research 59, 168-175.
Benech-Kieffer F, Meuling WJ, Leclerc C, Roza L, Leclaire J, & Nohynek G (2003). Percutaneous absorption of Mexoryl SX in human volunteers: comparison with in vitro data. Skin Pharmacol Appl Skin Physiol 16, 343-355.
Benfeldt E, Hansen SH, Volund A, Menne T, & Shah VP (2007). Bioequivalence of topical formulations in humans: evaluation by dermal microdialysis sampling and the dermatopharmacokinetic method. J Invest Dermatol 127, 170-178.
Benfeldt E & Serup J (1999). Effect of barrier perturbation on cutaneous penetration of salicylic acid in hairless rats: in vivo pharmacokinetics using microdialysis and non-invasive quantification of barrier function. Arch Dermatol Res 291, 517-526.
Benfeldt E, Serup J, & Menne T (1999). Effect of barrier perturbation on cutaneous salicylic acid penetration in human skin: in vivo pharmacokinetics using microdialysis and non-invasive quantification of barrier function. Br J Dermatol 140, 739-748.
Borras-Blasco J, ez-Sales O, Lopez A, & Herraez-Dominguez M (2004). A mathematical approach to predicting the percutaneous absorption enhancing effect of sodium lauryl sulphate. Int J Pharm 269, 121-129.
Bouwstra JA & Ponec M (2006). The skin barrier in healthy and diseased state. Biochimica et Biophysica Acta-Biomembranes 1758, 2080-2095.
Brain KR, Walters KA, & Watkinson AC (1998). Investigation of skin permeation in vitro. In Dermal Absorption and Toxicity Assessment, eds. Roberts MS & Walters KA, pp. 161-188. Marcel Dekker, New York.
Brand RM, Charron AR, Sandler VL, & Jendrzejewski JL (2007). Moisturizing lotions can increase transdermal absorption of the herbicide 2,4-dichlorophenoxacetic acid across hairless mouse skin. Cutan Ocul Toxicol 26, 15-23.
Brand RM & Mueller C (2002). Transdermal penetration of atrazine, alachlor, and trifluralin: effect of formulation. Toxicol Sci 68, 18-23.
Bronaugh RL (2004a). Methods for in vitro metabolism studies. In Dermatotoxicology, eds. Zhai H & Maibach HI, pp. 622-630. CRC Press, New York.
Bronaugh RL (2004b). Methods for in vitro percutaneous absorption. In Dermatotoxicology, eds. Zhai H & Maibach HI, pp. 520-526. CRC Press, New York.
Bronaugh RL & Maibach HI (1985). Percutaneous absorption of nitroaromatic compounds: in vivo and in vitro studies in the human and monkey. J Invest Dermatol 84, 180-183.
Bronaugh RL & Stewart RF (1985a). Methods for in vitro percutaneous absorption studies IV: The flow-through diffusion cell. J Pharm Sci 74, 64-67.
Bronaugh RL & Stewart RF (1985b). Methods for in vitro percutaneous absorption studies V: Permeation through damaged skin. J Pharm Sci 74, 1062-1066.
Bucks D, Maibach H, & Guy RH (1991). In Vitro Percutaneous Absorption: Pinciples, Fundamentals and Applications, pp. 85-114. CRC, Boca Raton, FL.
Buist HE, de HC, van Burgsteden JA, & van de Sandt JJ (2007). Dermatokinetics of didecyldimethylammonium chloride and the influence of some commercial biocidal formulations on its dermal absorption in vitro. Regul Toxicol Pharmacol 48, 87-92.
Buist HE, van de Sandt JJ, van Burgsteden JA, & de HC (2005). Effects of single and repeated exposure to biocidal active substances on the barrier function of the skin in vitro. Regul Toxicol Pharmacol 43, 76-84.
Campbell JL, Smith MA, Eiteman MA, Williams PL, & Boeniger MF (2000). Comparison of solvents for removing pesticides from skin using an in vitro porcine model. AIHAJ 61, 82-88.
Cessna AJ & Grover R (2002). Exposure of ground-rig applicators to the herbicide bromoxynil applied as a 1:1 mixture of butyrate and octanoate. Arch Environ Contam Toxicol 42, 369-382.
Chaurasia CS (1999). In vivo microdialysis sampling: theory and applications. Biomed Chromatogr 13, 317-332.
Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, Bungay PM, DeLange EC, Derendorf H, Elmquist WF, Hammarlund-Udenaes M, Joukhadar C, Kellogg DL, Jr., Lunte CE, Nordstrom CH, Rollema H, Sawchuk RJ, Cheung BW, Shah VP, Stahle L, Ungerstedt U, Welty DF, & Yeo H (2007). AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm Res 24, 1014-1025.
Chen H, Ho JCC, Sandilands A, Chan YC, Giam YC, Evans AT, Lane EB, & Mclean WHI (2008). Unique and recurrent mutations in the filaggrin gene in Singaporean Chinese patients with ichthyosis vulgaris. Journal of Investigative Dermatology 128, 1669-1675.
Chilcott RP, Barai N, Beezer AE, Brain SI, Brown MB, Bunge AL, Burgess SE, Cross S, Dalton CH, Dias M, Farinha A, Finnin BC, Gallagher SJ, Green DM, Gunt H, Gwyther RL, Heard CM, Jarvis CA, Kamiyama F, Kasting GB, Ley EE, Lim ST, McNaughton GS, Morris A, Nazemi MH, Pellett MA, Du PJ, Quan YS, Raghavan SL, Roberts M, Romonchuk W, Roper CS, Schenk D, Simonsen L, Simpson A, Traversa BD, Trottet L, Watkinson A, Wilkinson SC, Williams FM, Yamamoto A, & Hadgraft J (2005). Inter- and intralaboratory variation of in vitro diffusion cell measurements: an international multicenter study using quasi-standardized methods and materials. J Pharm Sci 94, 632-638.
Chilcott RP, Dalton CH, Emmanuel AJ, Allen CE, & Bradley ST (2002). Transepidermal water loss does not correlate with skin barrier function in vitro. Journal of Investigative Dermatology 118, 871-875.
Clark NWE (1992). Cutaneous xenobiotic metabolism and its role in percutaneous absorption, pp. 56-77.
Cnubben NH, Elliott GR, Hakkert BC, Meuling WJ, & van de Sandt JJ (2002). Comparative in vitro-in vivo percutaneous penetration of the fungicide ortho-phenylphenol. Regul Toxicol Pharmacol 35, 198-208.
Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, Duff GW, Ward SJ, & Tazi-Ahnini R (2006). New perspectives on epidermal barrier dysfunction in atopic dermatitis: Gene-environment interactions. Journal of Allergy and Clinical Immunology 118, 3-21.
Coronado GD, Thompson B, Strong L, Griffith WC, & Islas I (2004). Agricultural task and exposure to organophosphate pesticides among farmworkers. Environmental Health Perspectives 112, 142-147.
Coronado GD, Vigoren EM, Thompson B, Griffith WC, & Faustman EM (2006). Organophosphate pesticide exposure and work in pome fruit: Evidence for the take-home pesticide pathway. Environmental Health Perspectives 114, 999-1006.
Creely KS & Cherrie JW (2001). A novel method of assessing the effectiveness of protective gloves--results from a pilot study. Ann Occup Hyg 45, 137-143.
Cross SE, Magnusson BM, Winckle G, Anissimov Y, & Roberts MS (2003). Determination of the effect of lipophilicity on the in vitro permeability and tissue reservoir characteristics of topically applied solutes in human skin layers. J Invest Dermatol 120, 759-764.
Cross SE & Roberts MS (1993). Subcutaneous absorption kinetics and local tissue distribution of interferon and other solutes. J Pharm Pharmacol 45, 606-609.
Cua AB, Wilhelm KP, & Maibach HI (1995). Skin surface lipid and skin friction: relation to age, sex and anatomical region. Skin Pharmacol 8, 246-251.
Davies DJ, Ward RJ, & Heylings JR (2004). Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol In Vitro 18, 351-358.
de Cock J, Heederik D, Kromhout H, Boleij JSM, Hoek F, Wegh H, & Ny ET (1998a). Determinants of exposure to captan in fruit growing. American Industrial Hygiene Association Journal 59, 166-172.
de Cock J, Heederik D, Kromhout H, Boleij JSM, Hoek F, Wegh H, & Ny ET (1998b). Exposure to captan in fruit growing. American Industrial Hygiene Association Journal 59, 158-165.
de Jongh CM, Jakasa I, Verberk MM, & Kezic S (2006). Variation in barrier impairment and inflammation of human skin as determined by sodium lauryl sulphate penetration rate. British Journal of Dermatology 154, 651-657.
de Jongh CM, Khrenova L, Verbeck MM, Calkoen F, Dijk van FJH, Voss H, John SM, & Kezic S (2008). Loss-of-function polymorphisms in the filaggrin gene are associated with an increased susceptibility to chronic irritant contact dermatitis: a case-control study. Br J Dermatol 159, 621-627.
Denyer S.P, Guy RH, Hadgraft J, & Hugo W.B (1985). The microbial degradation of topically applied drugs. Int J Pharm 26, 89-97.
Dias M, Naik A, Guy RH, Hadgraft J, & Lane ME (2008). In vivo infrared spectroscopy studies of alkanol effects on human skin. Eur J Pharm Biopharm 69, 1171-1175.
Dickel H, Bruckner TM, Erdmann SM, Fluhr JW, Frosch PJ, Grabbe J, Loffler H, Merk HF, Pirker C, Schwanitz HJ, Weisshaar E, & Brasch J (2004). The "strip" patch test: results of a multicentre study towards a standardization. Arch Dermatol Res 296, 212-219.
Diepgen TL (2003). Occupational skin-disease data in Europe. Int Arch Occup Environ Health 76, 331-338.
Dooms-Goossens A, Deveylder H, de Alam AG, Lachapelle JM, Tennstedt D, & Degreef H (1989). Contact sensitivity to nonoxynols as a cause of intolerance to antiseptic preparations. J Am Acad Dermatol 21, 723-727.
DuPont Co Protective Apparel Fabrics of TYVEK. Permeation Guide for DuPont Protective Apparel Fabrics. 1993. Wilkington, DE.
Ref Type: Pamphlet
Dupuis D, Rougier A, Roguet R, & Lotte C (1986). The measurement of the stratum corneum reservoir: a simple method to predict the influence of vehicles on in vivo percutaneous absorption. Br J Dermatol 115, 233-238.
ECETOC. ECETOC 1993 Percutaneous abosrption. 20, 1-80. 1993. Brussels, European Centre for Ecotoxicology and Toxicology of Chemicals.
Ref Type: Report
Edwards JW, Lee SG, Heath LM, & Pisaniello DL (2007). Worker exposure and a risk assessment of Malathion and Fenthion used in the control of Mediterranean fruit fly in South Australia. Environmental Research 103, 38-45.
Effendy I & Maibach HI (1995). Surfactants and Experimental Irritant Contact-Dermatitis. Contact Dermatitis 33, 217-225.
Ehntholt DJ, Cerundolo DL, Bodek I, Schwope AD, Royer MD, & Nielsen AP (1990). A test method for the evaluation of protective glove materials used in agricultural pesticide operations. Am Ind Hyg Assoc J 51, 462-468.
Elias PM (1981). Epidermal lipids, membranes, and keratinization. Int J Dermatol 20, 1-19.
Elias PM (1983). Epidermal lipids, barrier function, and desquamation. J Invest Dermatol 80 Suppl, 44s-49s.
Elsner P (2007). Skin protection in the prevention of skin diseases. Curr Probl Dermatol 34, 1-10.
EPA. In vitro dermal absorption rate testing of certain chemicals of interest to the occupational safety and health administration. Federal Register: April 26, 2004 (Volume 69,Number 80), 1994; pp.22402-22442. 2004. 12-6-2008.
Ref Type: Internet Communication
Feldmann RJ & Maibach HI (1967). Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol 48, 181-183.
Fenske R, Curl C, Griffin S, Tchong M, & Galvin K (2006). Preventing work-to-home transmission of hazardous substances: A review of recent para-occupational exposure studies. Epidemiology 17, S169.
Fenske RA (2005). State-of-the-art measurement of agricultural pesticide exposures. Scandinavian Journal of Work Environment & Health 31, 67-73.
Fenske RA & Lu C (1994). Determination of handwash removal efficiency: incomplete removal of the pesticide chlorpyrifos from skin by standard handwash techniques. Am Ind Hyg Assoc J 55, 425-432.
Fenske RA, Schulter C, Lu C, & Allen EH (1998). Incomplete removal of the pesticide captan from skin by standard handwash exposure assessment procedures. Bulletin of Environmental Contamination and Toxicology 61, 194-201.
Fernandez C, Nielloud F, Fortune R, Vian L, & Marti-Mestres G (2002). Benzophenone-3: rapid prediction and evaluation using non-invasive methods of in vivo human penetration. J Pharm Biomed Anal 28, 57-63.
Fluhr JW, Feingold KR, & Elias PM (2006). Transepidermal water loss reflects permeability barrier status: validation in human and rodent in vivo and ex vivo models. Experimental Dermatology 15, 483-492.
Fluhr JW, Lazzerini S, Distante F, Gloor M, & Berardesca E (1999). Effects of prolonged occlusion on stratum corneum barrier function and water holding capacity. Skin Pharmacology and Applied Skin Physiology 12, 193-198.
Flynn GL (1990). Physicochemical determinants of skin absorption. In Principles of route to route extrapolation for risk assessment., eds. Gerrity TR & Henry CJ, pp. 93-127. Elsevir, New York.
Flyvholm MA, Bach B, Rose M, & Jepsen KF (2007). Self-reported hand eczema in a hospital population. Contact Dermatitis 57, 110-115.
Forslind B, Engstrom S, Engblom J, & Norlen L (1997). A novel approach to the understanding of human skin barrier function. J Dermatol Sci 14, 115-125.
Franz TJ (1975). Percutaneous absorption on the relevance of in vitro data. J Invest Dermatol 64, 190-195.
Frasch HF, Barbero AM, Alachkar H, & McDougal JN (2007). Skin penetration and lag times of neat and aqueous diethyl phthalate, 1,2-dichloroethane and naphthalene. Cutaneous and Ocular Toxicology 26, 147-160.
Gao P, El-Ayouby N, & Wassell JT (2005). Change in permeation parameters and the decontamination efficacy of three chemical protective gloves after repeated exposures to solvents and thermal decontaminations. Am J Ind Med 47, 131-143.
Garrod AN, Phillips AM, & Pemberton JA (2001). Potential exposure of hands inside protective gloves-a summary of data from non-agricultural pesticide surveys. Ann Occup Hyg 45, 55-60.
Gioia F & Celleno L (2002). The dynamics of transepidermal water loss (TEWL) from hydrated skin. Skin Res Technol 8, 178-186.
Grandjean P (1990). Skin Penetration - Hazardous Chemicals at Work Taylor and Francis.
Griffin P, Payne M, Mason H, Freedlander E, Curran AD, & Cocker J (2000). The in vitro percutaneous penetration of chlorpyrifos. Hum Exp Toxicol 19, 104-107.
Groth L (1996). Cutaneous microdialysis. Methodology and validation. Acta Derm Venereol Suppl (Stockh) 197, 1-61.
Guo C, Stone J, Stahr HM, & Shelley M (2001). Effects of exposure time, material type, and granular pesticide on glove contamination. Arch Environ Contam Toxicol 41, 529-536.
Guo C, Stone J, Stahr HM, & Shelley M (2002). Cleanup of gloves contaminated with granular terbufos and tefluthrin. Arch Environ Contam Toxicol 42, 383-388.
Guy RH, Hadgraft J, & Bucks DA (1987). Transdermal drug delivery and cutaneous metabolism. Xenobiotica 17, 325-343.
Guy RH, Hadgraft J, & Maibach HI (1985). Percutaneous absorption in man: a kinetic approach. Toxicol Appl Pharmacol 78, 123-129.
Guy RH & Potts RO (1993). Penetration of industrial chemicals across the skin: a predictive model. Am J Ind Med 23, 711-719.
Hadgraft J (2004). Skin deep. Eur J Pharm Biopharm 58, 291-299.
Halkier-Sorensen L (1996). Occupational skin diseases. Contact Dermatitis 35, 1-120.
Harville J & Que Hee SS (1989). Permeation of a 2,4-D isooctyl ester formulation through neoprene, nitrile, and Tyvek protection materials. Am Ind Hyg Assoc J 50, 438-446.
Hayes BB, Afshari A, Millecchia L, Willard PA, Povoski SP, & Meade BJ (2000). Evaluation of percutaneous penetration of natural rubber latex proteins. Toxicological Sciences 56, 262-270.
Hotchkiss SAM (1998). Dermal metabolism. In Dermal absorption and toxicity assessment., eds. Roberts MS & Walters KA, pp. 41-101. Marcel Dekker, New York.
Hueber-Becker F, Nohynek GJ, Dufour EK, Meuling WJA, De Bie ATHJ, Toutain H, & Bolt HM (2007). Occupational exposure of hairdressers to [C-14]-para-phenylenediamine-containing oxidative hair dyes: A mass balance study. Food and Chemical Toxicology 45, 160-169.
Hueber-Becker F, Nohynek GJ, Meuling WJ, ech-Kieffer F, & Toutain H (2004). Human systemic exposure to a [14C]-para-phenylenediamine-containing oxidative hair dye and correlation with in vitro percutaneous absorption in human or pig skin. Food Chem Toxicol 42, 1227-1236.
Idson B (1971). Biophysical Factors in Skin Penetration. Journal of the Society of Cosmetic Chemists 22, 615-622.
Ilyin LA, Ivannikov AT, Parfenov YD, & Stolyarov VP (1975). Strontium absorption through damaged and undamaged human skin. Health Phys 29, 75-80.
Imokawa G (2001). Lipid abnormalities in atopic dermatitis. Journal of the American Academy of Dermatology 45, S29-S32.
Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, & Hidano A (1991). Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol 96, 523-526.
IPCS (2006). Dermal Absorption - Environmental Health Criteria 235 WHO.
IPCS INCHEM. Naphthalene. International Programme on Chemical Safety . 2002. 12-6-2008.
Ref Type: Internet Communication
Jacobi U, Weigmann HJ, Baumann M, Reiche AI, Sterry W, & Lademann J (2004). Lateral spreading of topically applied UV filter substances investigated by tape stripping. Skin Pharmacol Physiol 17, 17-22.
Jakasa I, de Jongh CM, Verberk MM, Bos JD, & Kezic S (2006a). Percutaneous penetration of sodium lauryl sulphate is increased in uninvolved skin of patients with atopic dermatitis compared with control subjects. Br J Dermatol 155, 104-109.
Jakasa I, Mohammadi N, Kruse J, & Kezic S (2004). Percutaneous absorption of neat and aqueous solutions of 2-butoxyethanol in volunteers. Int Arch Occup Environ Health 77, 79-84.
Jakasa I, Verberk MM, Bunge AL, Kruse J, & Kezic S (2006b). Increased permeability for polyethylene glycols through skin compromised by sodium lauryl sulphate. Exp Dermatol 15, 801-807.
Jakasa I, Verberk MM, Esposito M, Bos JD, & Kezic S (2007). Altered penetration of polyethylene glycols into uninvolved skin of atopic dermatitis patients. J Invest Dermatol 127, 129-134.
Johnson JM, Brengelmann GL, Hales JR, Vanhoutte PM, & Wenger CB (1986). Regulation of the cutaneous circulation. Fed Proc 45, 2841-2850.
Jones K, Cocker J, Dodd LJ, & Fraser I (2003). Factors affecting the extent of dermal absorption of solvent vapours: A human volunteer study. Annals of Occupational Hygiene 47, 145-150.
Kamijima M, Hisanaga N, Wang HL, & Nakajima T (2007). Occupational trichloroethylene exposure as a cause of idiosyncratic generalized skin disorders and accompanying hepatitis similar to drug hypersensitivities. International Archives of Occupational and Environmental Health 80, 357-370.
Kao J, Hall J, & Helman G (1988). In vitro percutaneous absorption in mouse skin: influence of skin appendages. Toxicol Appl Pharmacol 94, 93-103.
Karr C, Demers P, Costa LG, Daniell WE, Barnhart S, Miller M, Gallagher G, Horstman SW, Eaton D, & Rosenstock L (1992). Organophosphate Pesticide Exposure in A Group of Washington-State Orchard Applicators. Environmental Research 59, 229-237.
Kasting GB, Smith RL, & Anderson BD (1992). Prodrugs for dermal delivery; solubility, molecular size and functional group effects., pp. 117-161. Marcel Dekker, New York.
Kasting GB, Smith RL, & Cooper ER (1987). Effect of lipid solubility and molecular size on percutaneous absorption. In Skin pharmacokinetics, eds. Shroot B & Schaefer H, pp. 138-153. Karger, Basel.
Keeble VB, Correll L, & Ehrich M (1993). Evaluation of knit glove fabrics as barriers to dermal absorption of organophosphorus insecticides using an in vitro test system. Toxicology 81, 195-203.
Keeble VB, Correll L, & Ehrich M (1996). Effect of laundering on ability of glove fabrics to decrease the penetration of organophosphate insecticides through in vitro epidermal systems. J Appl Toxicol 16, 401-406.
Keith LH, Conoly M, Nolen RL, Walters DB, & Prokopetz AT. Chemical permeation and degradation data from the National Toxicology Program measured by Radian Corporation. 1000.
Ref Type: Video Recording
Kezic S, Kemperman PM, Koster ES, de Jongh CM, Thio HB, Campbell LE, Irvine AD, McLean IW, Puppels GJ, & Caspers PJ (2008). Loss-of-Function Mutations in the Filaggrin Gene Lead to Reduced Level of Natural Moisturizing Factor in the Stratum Corneum. J Invest Dermatol128, 2117-2119.
Kezic S, Monster AC, van dG, I, Kruse J, Opdam JJ, & Verberk MM (2001). Dermal absorption of neat liquid solvents on brief exposures in volunteers. AIHAJ 62, 12-18.
Kezic S & Nielsen JB (2008). Absorption of chemicals through compromised skin. International Archives of Occupational and Environmental Health, in press.
Kirknel E & Sjelborg P. Handskers beskyttelsesevne ved arbejde med pesticider i jordbrugene, samt modeller for håndeksponering. 73. 2003. Danish Environment Protection Agency, Copenhagen, Denmark.
Ref Type: Report
Klaassen CD (1996). Casarett & Doull's Toxicology, 5th ed., pp. 529-546. McGraw-Hill, New York.
Knepp VM, Hadgraft J, & Guy RH (1987). Transdermal drug delivery: problems and possibilities. Crit Rev Ther Drug Carrier Syst 4, 13-37.
Korinth G, Geh S, Schaller KH, & Drexler H (2003a). In vitro evaluation of the efficacy of skin barrier creams and protective gloves on percutaneous absorption of industrial solvents. International Archives of Occupational and Environmental Health 76, 382-386.
Korinth G, Goen T, Koch HM, Merz T, & Uter W (2005). Visible and subclinical skin changes in male and female dispatch department workers of newspaper printing plants. Skin Res Technol 11, 132-139.
Korinth G, Goen T, Lakemeyer M, Broding HC, & Drexler H (2003b). Skin strain and its influence on systemic exposure to a glycol ether in offset printing workers. Contact Dermatitis 49, 248-254.
Korinth G, Goen T, Schaller KH, & Drexler H (2007a). Discrepancies between different rat models for the assessment of percutaneous penetration of hazardous substances. Archives of Toxicology 81, 833-840.
Korinth G, Jakasa I, Wellner T, Kezic S, Kruse J, & Schaller KH (2007b). Percutaneous absorption and metabolism of 2-butoxyethanol in human volunteers: a microdialysis study. Toxicol Lett 170, 97-103.
Korinth G, Weiss T, Penkert S, Schaller KH, Angerer J, & Drexler H (2007c). Percutaneous absorption of aromatic amines in rubber industry workers: impact of impaired skin and skin barrier creams. Occup Environ Med 64, 366-372.
Kreilgaard M (2002). Assessment of cutaneous drug delivery using microdialysis. Adv Drug Deliv Rev 54 Suppl 1, S99-S121.
Krishnaiah YS, Kumar MS, Raju V, Lakshmi M, & Rama B (2008). Penetration-enhancing effect of ethanolic solution of menthol on transdermal permeation of ondansetron hydrochloride across rat epidermis. Drug Deliv 15, 227-234.
Krzeminska S & Szczecinska K (2001). Proposal for a method for testing resistance of clothing and gloves to penetration by pesticides. Ann Agric Environ Med 8, 145-150.
Lammers JHCM, Meuling WJ, Muijser H, Freidig AP, & Bessems JGM (2005). Neurobehavioural evaluation and kinetics of inhalation of constant or fluctuating toluene concentrations in human volunteers. Environ Toxicol Pharmacol 20, 431-442.
Larson E, Friedman C, Cohran J, Treston-Aurand J, & Green S (1997). Prevalence and correlates of skin damage on the hands of nurses. Heart Lung 26, 404-412.
Lboutounne H, Chaulet JF, Ploton C, Falson F, & Pirot F (2002). Sustained ex vivo skin antiseptic activity of chlorhexidine in poly(epsilon-caprolactone) nanocapsule encapsulated form and as a digluconate. J Control Release 82, 319-334.
Lee FW. In vitro percutaneous absorption of lipophilic chemicals. 2000.
Ref Type: Report
Levang AK, Zhao K, & Singh J (1999). Effect of ethanol/propylene glycol on the in vitro percutaneous absorption of aspirin, biophysical changes and macroscopic barrier properties of the skin. Int J Pharm 181, 255-263.
Levin J & Maibach H (2005). The correlation between transepidermal water loss and percutaneous absorption: an overview. J Control Release 103, 291-299.
Liu KH & Kim JH (2003). In vitro dermal penetration study of carbofuran, carbosulfan, and furathiocarb. Arch Toxicol 77, 255-260.
Liu KH, Sung HJ, Lee HK, Song BH, Ihm YB, Kim K, Lee HS, & Kim JH (2002). Dermal pharmacokinetics of the insecticide furathiocarb in rats. Pest Manag Sci 58, 57-62.
Loffler H, Dreher F, & Maibach HI (2004). Stratum corneum adhesive tape stripping: influence of anatomical site, application pressure, duration and removal. Br J Dermatol 151, 746-752.
Lonnroth P, Jansson PA, & Smith U (1987). A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol 253, E228-E231.
Machera K, Goumenou M, Kapetanakis E, Kalamarakis A, & Glass CR (2003). Determination of potential dermal and inhalation operator exposure to malathion in greenhouses with the whole body dosimetry method. Annals of Occupational Hygiene 47, 61-70.
Magnusson BM, Anissimov YG, Cross SE, & Roberts MS (2004). Molecular size as the main determinant of solute maximum flux across the skin. J Invest Dermatol 122, 993-999.
Maibach H & Patrick E (2001). Dermatotoxicology. In Principles and methods of toxicology pp. 1039-1046. Taylor and Francis.
McCarley KD & Bunge AL (2003). Absorption into silicone rubber membranes from powders and aqueous solutions. International Journal of Pharmaceutics 250, 169-180.
McDougal JN & Boeniger MF (2002). Methods for assessing risks of dermal exposures in the workplace. Critical Reviews in Toxicology 32, 291-327.
McKone TE & Howd RA (1992). Estimating dermal uptake of nonionic organic chemicals from water and soil: I. Unified fugacity-based models for risk assessments. Risk Anal 12, 543-557.
Meding B, Lantto R, Lindahl G, Wrangsjo K, & Bengtsson B (2005). Occupational skin disease in Sweden - a 12-year follow-up. Contact Dermatitis 53, 308-313.
Meuling WJA, Franssen AC, Brouwer DH, & vanHemmen JJ (1997). The influence of skin moisture on the dermal absorption of propoxur in human volunteers: A consideration for biological monitoring practices. Science of the Total Environment 199, 165-172.
Michales AS, Chandrasekaran SK, & Shaw JE (1975). Drug permeation through human skin: Theory and in vitro experimental measurement. AIChE Journal 21, 985-996.
Miljøstyrelsen. Bekæmpelsesmiddelstatistik 1997. 1998. Miljøstyrelsen, 221 Miljøstyrelsen. 6-1-2008.
Ref Type: Report
Miljøstyrelsen. Bekæmpelsesmiddelstatistik 2006. Orientering fra Miljøstyrelsen Nr. 5 2007. 2007. Miljøstyrelsen. 6-1-2008.
Ref Type: Report
Miller G (2002). Living in the Environment, 12 ed. Belmont: Wadsworth/Thomson Learning..
Miller G (2004). Sustaining the Earth. pp. 211-216. Thompson Learning, Inc., Pacific Grove, CA.
Mills PC (2007). Vehicle effects on the in vitro penetration of testosterone through equine skin. Veterinary Research Communications 31, 227-233.
Mills PC, Magnusson BM, & Cross SE (2005). Effects of vehicle and region of application on absorption of hydrocortisone through canine skin. American Journal of Veterinary Research 66, 43-47.
Mills PC, Magnusson BM, & Cross SE (2006). The effects of vehicle and region of application on in vitro penetration of testosterone through canine skin. Veterinary Journal 171, 276-280.
Moody RP & Nadeau B (1994). Nitrile butyl rubber glove permeation of pesticide formulations containing 2,4-D-amine, DDT, DEET, and Diazinon. Bull Environ Contam Toxicol 52, 125-130.
Moody RP & Ritter L (1990). Pesticide glove permeation analysis: comparison of the ASTM F739 test method with an automated flow-through reverse-phase liquid chromatography procedure. Am Ind Hyg Assoc J 51, 79-83.
Morgan CJ, Renwick AG, & Friedmann PS (2003). The role of stratum corneum and dermal microvascular perfusion in penetration and tissue levels of water-soluble drugs investigated by microdialysis. Br J Dermatol 148, 434-443.
Muller M (2002). Science, medicine, and the future: Microdialysis. BMJ 324, 588-591.
nationmaster. Encyclopedia > Pesticide. nationmaster. NationMaster.com . 2003. 6-1-2008.
Ref Type: Electronic Citation
Nielsen GD, Nielsen JB, Andersen KE, & Grandjean P (2000). Effects of industrial detergents on the barrier function of human skin. Int J Occup Environ Health 6, 138-142.
Nielsen JB. Hudpenetration af pesticider - en undersøgelse af effekten af hjælpestoffer, kombinationseffekter, handsker, samt lettere beskadiget hud. [90], 1-62. 2004. Bekæmpelsesmiddelforskning fra Miljøstyrelsen, Miljøstyrelsen.
Nielsen JB (2005b). The selection and use of gloves against pesticides. In Protective gloves for occupational use, eds. Boman A, Estlander T, Wahlberg JE, & Maibach HI, pp. 321-334. CRC Press.
Nielsen JB, Nielsen F, & Sørensen JA. Hudoptagelse af pesticider - betydning af lag-time og reservoir effekt. 2006. Miljøstyrelsen. Bekæmpelsesmiddelforskning fra Miljøstyrelsen.
Nielsen JB (2000). Effects of four detergents on the in-vitro barrier function of human skin. Int J Occup Environ Health 6, 143-147.
Nielsen JB (2005a). Percutaneous penetration through slightly damaged skin. Arch Dermatol Res 296, 560-567.
Nielsen JB & Andersen HR (2001). Dermal in vitro penetration of methiocarb, paclobutrazol, and pirimicarb: effect of nonylphenolethoxylate and protective gloves. Environ Health Perspect 109, 129-132.
Nielsen JB & Nielsen F (2000). Dermal in vitro penetration of methiocarb, paclobutrazol, and pirimicarb. Occup Environ Med 57, 734-737.
Nielsen JB, Nielsen F, & Sorensen JA (2004). In vitro percutaneous penetration of five pesticides--effects of molecular weight and solubility characteristics. Ann Occup Hyg 48, 697-705.
Nielsen JB, Nielsen F, & Sorensen JA (2007). Defense against dermal exposures is only skin deep: significantly increased penetration through slightly damaged skin. Arch Dermatol Res 299, 423-431.
Nohynek GJ, Meuling WJ, Vaes WH, Lawrence RS, Shapiro S, Schulte S, Steiling W, Bausch J, Gerber E, Sasa H, & Nau H (2006). Repeated topical treatment, in contrast to single oral doses, with Vitamin A-containing preparations does not affect plasma concentrations of retinol, retinyl esters or retinoic acids in female subjects of child-bearing age. Toxicol Lett 163, 65-76.
Nohynek GJ, Skare JA, Meuling WJ, Hein DW, De Bie AT, & Toutain H (2004). Urinary acetylated metabolites and N-acetyltransferase-2 genotype in human subjects treated with a para-phenylenediamine-containing oxidative hair dye. Food Chem Toxicol 42, 1885-1891.
Nokhodchi A, Shokri J, Dashbolaghi A, Hassan-Zadeh D, Ghafourian T, & Barzegar-Jalali M (2003). The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int J Pharm 250, 359-369.
Nomura T, Sandilands A, Akiyama M, Liao HH, Evans AT, Sakai K, Ota M, Sugiura H, Yamamoto K, Sato H, Palmer CNA, Smith FJD, Mclean WHI, & Shimizu H (2007). Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. Journal of Allergy and Clinical Immunology 119, 434-440.
Obata Y, Takayama K, Maitani Y, Machida Y, & Nagai T (1993). Effect of Ethanol on Skin Permeation of Nonionized and Ionized Diclofenac. International Journal of Pharmaceutics 89, 191-198.
OECD. OECD-428 Guidance document for the conduct of skin absorption studies. OECD environment health and safety publications; series on testing and assessment#28. 2000. Paris: Organisation for Economic Cooperation and Development.
Ref Type: Report
OECD. OECD (2004a) Guidance document for the conduct of skin absorption studies. Paris. 28, 1-31. 2004.
Ref Type: Report
Okuda M, Yoshiike T, & Ogawa H (2002). Detergent-induced epidermal barrier dysfunction and its prevention. Journal of Dermatological Science 30, 173-179.
Packham C (2006). Gloves as chemical protection - Can they really work? Annals of Occupational Hygiene 50, 545-548.
Palenske J & Morhenn VB (1999). Changes in the skin's capacitance after damage to the stratum corneum in humans. J Cutan Med Surg 3, 127-131.
Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O'Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El HB, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, & McLean WH (2006). Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 38, 441-446.
Pershing LK, Corlett J, & Jorgensen C (1994). In vivo pharmacokinetics and pharmacodynamics of topical ketoconazole and miconazole in human stratum corneum. Antimicrob Agents Chemother 38, 90-95.
Phalen R & Hee SQ (2008). A moving robotic hand system for whole-glove permeation and penetration: Captan and nitrile gloves. Journal of Occupational and Environmental Hygiene 5, 258-270.
Pilgram GS, Vissers DC, van der MH, Pavel S, Lavrijsen SP, Bouwstra JA, & Koerten HK (2001). Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol 117, 710-717.
Piret J, Desormeaux A, Cormier H, Lamontagne J, Gourde P, Juhasz J, & Bergeron MG (2000). Sodium lauryl sulfate increases the efficacy of a topical formulation of foscarnet against herpes simplex virus type 1 cutaneous lesions in mice. Antimicrob Agents Chemother 44, 2263-2270.
Plezia PM, Kramer TH, Linford J, & Hameroff SR (1989). Transdermal fentanyl: pharmacokinetics and preliminary clinical evaluation. Pharmacotherapy 9, 2-9.
Potts RO & Guy RH (1992). Predicting skin permeability. Pharm Res 9, 663-669.
Proksch E, Brasch J, & Sterry W (1996). Integrity of the permeability barrier regulates epidermal Langerhans cell density. Br J Dermatol 134, 630-638.
Proksch E, Jensen JM, & Elias PM (2003). Skin lipids and epidermal differentiation in atopic dermatitis. Clinics in Dermatology 21, 134-144.
Purdham JT, Menard BJ, Bozek PR, & Sass-Kortsak AM (2001). MCPA permeation through protective gloves. Appl Occup Environ Hyg 16, 961-966.
Raheel M & Dai GX (1997). Chemical resistance and structural integrity of protective glove materials. j Environ Sci Health A23.
Raheel M & Dai GX (2002). Viability of textile systems for hand and body protection: effects of chemical interaction, wear, and storage conditions. Bull Environ Contam Toxicol 69, 164-172.
Ramsey JD, Woollen BH, Auton TR, & Scott RC (1994). The predictive accuracy of in vitro measurements for the dermal absorption of a lipophilic penetrant (fluazifop-butyl) through rat and human skin. Fundam Appl Toxicol 23, 230-236.
Rawlings AV & Harding CR (2004). Moisturization and skin barrier function. Dermatol Ther 17 Suppl 1, 43-48.
Rawson BV, Cocker J, Evans PG, Wheeler JP, & Akrill PM (2005). Internal contamination of gloves: Routes and consequences. Annals of Occupational Hygiene 49, 535-541.
Reddy MB, Stinchcomb AL, Guy RH, & Bunge AL (2002). Determining dermal absorption parameters in vivo from tape strip data. Pharm Res 19, 292-298.
Ribaud C, Garson JC, Doucet J, & Leveque JL (1994). Organization of stratum corneum lipids in relation to permeability: influence of sodium lauryl sulfate and preheating. Pharm Res 11, 1414-1418.
Roberts MS & Walters KA (1998a). Dermal Absorption and Toxicity Assessment Marcel Dekker, Inc, New York.
Roberts MS & Walters KA (1998b). Drugs and the Pharmacentical Sciences. In Dermal Absorption and Toxicity Assessment pp. 1-42. Marcel Dekker Inc., New York.
Roberts MS, Cross SE, & Anissimov YG (2004). Factors affecting the formation of a skin reservoir for topically applied solutes. Skin Pharmacol Physiol 17, 3-16.
Rogers J, Harding C, Mayo A, Banks J, & Rawlings A (1996). Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res 288, 765-770.
Romonchuk WJ & Bunge AL (2006). Permeation of 4-cyanophenol and methyl paraben from powder and saturated aqueous solution through silicone rubber membranes and human skin. Journal of Pharmaceutical Sciences 95, 2526-2533.
Rosado C & Rodrigues LM (2003). Solvent effects in permeation assessed in vivo by skin surface biopsy. BMC Dermatol 3, 5.
Roskos KV, Maibach HI, & Guy RH (1989). The effect of aging on percutaneous absorption in man. J Pharmacokinet Biopharm 17, 617-630.
Rougier A, Dupuis D, Lotte C, & Maibach HI (1999). Stripping method for measuring percutaneous absorption in vivo. In Percutaneous absorption: drugs-cosmetics-mechanisms-methodology pp. 375-393. Marcel Dekker, New York.
Rowse DH & Emmett EA (2004). Solvents and the skin. Clin Occup Environ Med 4, 657-730, vi.
Roy SD & Flynn GL (1989). Transdermal delivery of narcotic analgesics: comparative permeabilities of narcotic analgesics through human cadaver skin. Pharm Res 6, 825-832.
Roy SD, Hou SY, Witham SL, & Flynn GL (1994). Transdermal delivery of narcotic analgesics: comparative metabolism and permeability of human cadaver skin and hairless mouse skin. J Pharm Sci 83, 1723-1728.
Safety 4 (1993). 4H Chemical Protective Guide Safety 4 a/s, Lyngby, Denmark.
Sartorelli P, Andersen HR, Angerer J, Corish J, Drexler H, Goen T, Griffin P, Hotchkiss SA, Larese F, Montomoli L, Perkins J, Schmelz M, van de SJ, & Williams F (2000). Percutaneous penetration studies for risk assessment. Environ Toxicol Pharmacol 8, 133-152.
Sartorelli P, Aprea C, Bussani R, Novelli MT, Orsi D, & Sciarra G (1997). In vitro percutaneous penetration of methyl-parathion from a commercial formulation through the human skin. Occup Environ Med 54, 524-525.
Sarveiya V, Risk S, & Benson HA (2004). Liquid chromatographic assay for common sunscreen agents: application to in vivo assessment of skin penetration and systemic absorption in human volunteers. J Chromatogr B Analyt Technol Biomed Life Sci 803, 225-231.
Sato M, Fukayo S, & Yano E (2003). Adverse environmental health effects of ultra-low relative humidity indoor air. J Occup Health 45, 133-136.
Scheuplein RJ & Blank IH (1971). Permeability of the skin. Physiol Rev 51, 702-747.
Scheuplein RJ & Bronaugh RL (1983). Percutaneous absorption. In Biochemistry and physiology of the skin, ed. Goldsmith LA, pp. 1255-1295. Oxford University Press, Oxford.
Schreiner V, Gooris GS, Pfeiffer S, Lanzendorfer G, Wenck H, Diembeck W, Proksch E, & Bouwstra J (2000). Barrier characteristics of different human skin types investigated with X-ray diffraction, lipid analysis, and electron microscopy imaging. J Invest Dermatol 114, 654-660.
Schwope AD, Goydan R, Ehntholt D, Frank U, & Nielsen A (1992). Permeation resistance of glove materials to agricultural pesticides. Am Ind Hyg Assoc J 53, 352-361.
Scott RC, Batten PL, Clowes HM, Jones BK, & Ramsey JD (1992). Further validation of an in vitro method to reduce the need for in vivo studies for measuring the absorption of chemicals through rat skin. Fundam Appl Toxicol 19, 484-492.
Scott RC & Dugard PH (1986). A model for quantifying absorption through abnormal skin. J Invest Dermatol 86, 208-212.
Scott RC, Dugard PH, & Doss AW (1986). Permeability of abnormal rat skin. J Invest Dermatol 86, 201-207.
Seidenari S & Giusti G (1995). Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol 75, 429-433.
Silkowski JB, Horstman SW, & Morgan MS (1984). Permeation through five commercially available glove materials by two pentachlorophenol formulations. Am Ind Hyg Assoc J 45, 501-504.
Simcox NJ, Camp J, Kalman D, Stebbins A, Bellamy G, Lee IC, & Fenske R (1999). Farmworker exposure to organophosphorus pesticide residues during apple thinning in Central Washington State. American Industrial Hygiene Association Journal 60, 752-761.
Singer MM & Tjeerdema RS (1993). Fate and effects of the surfactant sodium dodecyl sulfate. Rev Environ Contam Toxicol 133, 95-149.
Sjelborg P, Kirknel E, Kristensen K, & Laursen BB. Modellering af pesticideksponering i danske frugtplantager og væksthuse samt værnemidlers effektivitet. 2008. Miljøstyrelsen.
Ref Type: Report
Stahl M, Bouw R, Jackson A, & Pay V (2002). Human microdialysis. Curr Pharm Biotechnol 3, 165-178.
Stone J, Coffman C, Imerman PM, Song K, & Shelley M (2005). Cotton liners to mediate glove comfort for greenhouse applicators. Arch Environ Contam Toxicol 49, 421-428.
Strong LL, Thompson B, Coronado GD, Griffith WC, Vigoren EM, & Islas I (2004). Health symptoms and exposure to organophosphate pesticides in farmworkers. American Journal of Industrial Medicine 46, 599-606.
Suuronen K, alto-Korte K, Piipari R, Tuomi T, & Jolanki R (2007). Occupational dermatitis and allergic respiratory diseases in Finnish metalworking machinists. Occupational Medicine-Oxford 57, 277-283.
Takahashi K, Tamagawa S, Katagi T, Yoshitomi H, Kamada A, Rytting JH, Nishihata T, & Mizuno N (1991). Invitro Transport of Sodium Diclofenac Across Rat Abdominal Skin - Effect of Selection of Oleaginous Component and the Addition of Alcohols to the Vehicle. Chemical & Pharmaceutical Bulletin 39, 154-158.
Thestrup-Pedersen K, Andersen KE, & Zachariae H (1993). Klinisk dermatologi og Venerologi Munksgaard.
Treffel P & Gabrad B (1996). Vehicle influence on the in vitro skin penetration of ultra-violet filters used in sun screen formulations. In Prediction of percutaneous penetration pp. 178-181. STS Publishing Ltd. Cardiff.
Tsai JC, Shen LC, Sheu HM, & Lu CC (2003). Tape stripping and sodium dodecyl sulfate treatment increase the molecular weight cutoff of polyethylene glycol penetration across murine skin. Arch Dermatol Res 295, 169-174.
Tsai JC, Sheu HM, Hung PL, & Cheng CL (2001). Effect of barrier disruption by acetone treatment on the permeability of compounds with various lipophilicities: implications for the permeability of compromised skin. J Pharm Sci 90, 1242-1254.
TupkerRA. The influence of detergent on human skin. 1-140. 1990. University of Groningen, Netherlands.
Ref Type: Thesis/Dissertation
Ungerstedt U. Measurement of Neurotransmitter Release by Intracranial Dialysis. 81-105. 1984. Marsden CA.
Ref Type: Report
US Environmental Protection Agency. Pesticide Worker Protection Standard; Glove Liners, and Chemical-Resistant Glove Requirements for Agricultural Pilots. 2004. 10-7-2008.
Ref Type: Internet Communication
US Environmental Protection Agency. US Environmental Protection Agency. 2007. 12-12-2007.
Ref Type: Internet Communication
Vallet V, Cruz C, Josse D, Bazire A, Lallement G, & Boudry I (2007). In vitro percutaneous penetration of organophosphorus compounds using full-thickness and split-thickness pig and human skin. Toxicology in Vitro 21, 1182-1190.
van de Sandt JJ, van Burgsteden JA, Cage S, Carmichael PL, Dick I, Kenyon S, Korinth G, Larese F, Limasset JC, Maas WJ, Montomoli L, Nielsen JB, Payan JP, Robinson E, Sartorelli P, Schaller KH, Wilkinson SC, & Williams FM (2004). In vitro predictions of skin absorption of caffeine, testosterone, and benzoic acid: a multi-centre comparison study. Regul Toxicol Pharmacol 39, 271-281.
van Ravenzwaay B. & Leibold E (2004). A comparison between in vitro rat and human and in vivo rat skin absorption studies. Hum Exp Toxicol 23, 421-430.
Varvel JR, Shafer SL, Hwang SS, Coen PA, & Stanski DR (1989). Absorption characteristics of transdermally administered fentanyl. Anesthesiology 70, 928-934.
Vasilopoulos Y, Cork MJ, Murphy R, Williams HC, Robinson DA, Duff GW, Ward SJ, & Tazi-Ahnini R (2004). Genetic association between an AACC insertion in the 3 ' UTR of the stratum corneum chymotryptic enzyme gene and atopic dermatitis. Journal of Investigative Dermatology 123, 62-66.
Vecchia BE & Bunge AL (2003). Evaluating the transdermal permeability of chemicals. In Transdermal drug delivery, eds. Guy RH & Hadgraft J., pp. 25-55. Marcel Dekker, New York.
Vela-Acosta MS, Bigelow P, & Buchan R (2002). Assessment of occupational health and safety risks of farmworkers in Colorado. American Journal of Industrial Medicine 19-27.
Vickers CF (1972). Stratum corneum reservoir for drugs. Adv Biol Skin 12, 177-189.
Waller JM & Maibach HI (2006). Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol 12, 145-154.
Walters KA & Roberts MS (1993). Veterinary applications of skin penetration enhancers. In Pharmaceutical skin penetration enhancement pp. 345-364. Marcel Dekker, New York.
Warner RR, Stone KJ, & Boissy YL (2003). Hydration disrupts human stratum corneum ultrastructure. Journal of Investigative Dermatology 120, 275-284.
Wester RC & Maibach HI (1983). Cutaneous pharmacokinetics: 10 steps to percutaneous absorption. Drug Metab Rev 14, 169-205.
Wilkinson SC, Maas WJ, Nielsen JB, Greaves LC, van de Sandt JJ, & Williams FM (2006). Interactions of skin thickness and physicochemical properties of test compounds in percutaneous penetration studies. Int Arch Occup Environ Health 79, 405-413.
Williams AC & Barry BW (2004). Penetration enhancers. Advanced Drug Delivery Reviews 56, 603-618.
Wilschut A, ten Berge WF, Robinson PJ, & McKone TE (1995). Estimating skin permeation. The validation of five mathematical skin permeation models. Chemosphere 30, 1275-1296.
Wissing SA & Muller RH (2002). Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. J Control Release 81, 225-233.
Wurster DE & Kramer SF (1961). Investigation of Some Factors Influencing Percutaneous Absorption. Journal of Pharmaceutical Sciences 50, 288-293.
Yamamoto A, Serizawa S, Ito M, & Sato Y (1991). Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res 283, 219-223.
Zendzian RP (2003). Pesticide residue on/in the washed skin and its potential contribution to dermal toxicity. J Appl Toxicol 23, 121-136.
Zhai HB & Maibach HI (2001). Effects of skin occlusion on percutaneous absorption: An overview. Skin Pharmacology and Applied Skin Physiology 14, 1-10.
Version 1.0 May 2009, © Danish Environmental Protection Agency